The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle. The solute is the substance that is dissolved in the solvent. The amount of solute that.
Aqueous Solution Science flashcards, Flashcards, Science
A set of values of the variables that satisfies an equation.
An aqueous solution of a volatile substance, a water (aqua);
Air, for example, is a solution consisting chiefly of oxygen and nitrogen with trace amounts of. An action or process of solving a problem. What is a solution in science grade 6? What is a solution in science?
In a solution, all the components appear as a single phase.
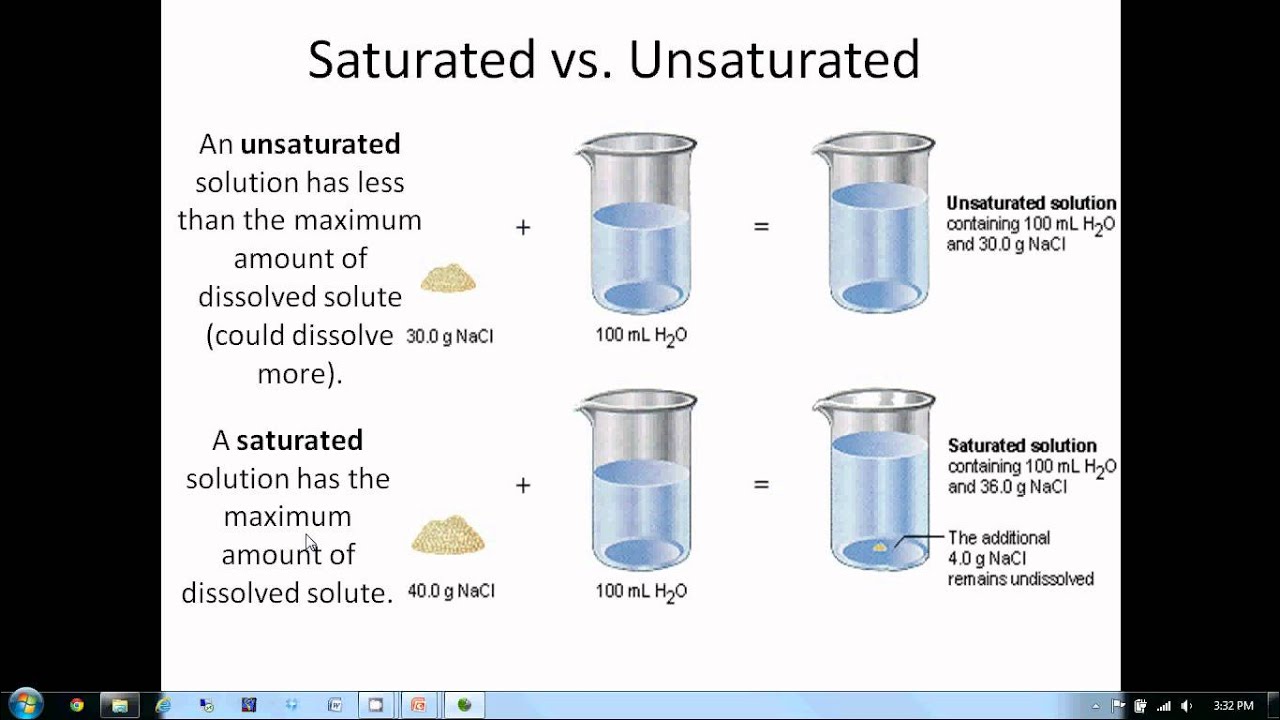
A minor disturbance of the solution or introduction of a seed or tiny crystal of solute will force crystallization of excess solute. Some examples of solutions include seawater, gasoline, glass, steel, and air. A solution is the same, or uniform, throughout which makes it a homogeneous mixture. Some solutions are combinations of two or more gases, or two or more liquids or even two or more solids.
A solution may exist in any phase.
Our books collection hosts in multiple countries, allowing you to get the most less latency time to download any of our books like this one. A solution is a homogeneous mixture of two or more substances. For solutions with components in the same phase, the substances present in lower concentration are solutes, while the substance present in highest abundance is the solvent. In scientific language, a solution is a mixture of at least two substances in which the particles of the substances are of atomic or molecular size.
Technically speaking, a solution consists of a mixture of one or more solutes dissolved in a solvent.
A solvent is a substance that dissolves another substance by pulling the molecules apart through electrochemical interactions. A solution is a homogeneous mixture of solvent and solute molecules. The solute then diffuses through the solvent until the concentration is equal in all parts of the solution. A solution consists of a solute and a solvent.
And an alcoholic solution of a volatile substance, a spirit (spiritus).
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. Most solutions are made when more than one gas, solid or liquid is dissolved in a liquid. Go here to learn more about mixtures. The definition of a supersaturated solution is one which contains more dissolved solute than could ordinarily dissolve into the solvent.
This involves transforming the defined logical decomposition models and their associated sets of derived.
A solution is a homogeneous mixture of two or more substances. A solution is a specific type of mixture where one substance is dissolved into another. An answer to a problem : One way supersaturation can occur is by carefully cooling a saturated solution.
Saturation is a physical or chemical situation where a system can take no more of a substance.
Saturation occurs in many different areas of science. The concentration of a solute in a solution is a measure of how much of that solute is dissolved in the solvent, with regard to how much solvent is present like salt. Science solutions definition is available in our book collection an online access to it is set as public so you can get it instantly. An alcoholic solution of a nonvolatile substance, a tincture (tinctura);
Generally, an aqueous solution of a nonvolatile substance called a solution or liquor;
A homogeneous mixture composed of two or more substances, in which a mixture, a solute is a substance dissolved in another substance known as a solvent. The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. One example of a solution is salt water which is a mixture of water and salt.
Substances that are combined to form a solution do not change into new substances.
A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. A solution consists of a solute and a solvent. The substance that dissolves in a solvent to produce a homogeneous mixture. A solution may exist in any phase.
Technically speaking, a solution consists of a mixture of one or more solutes dissolved in a solvent.
The term solution is commonly applied to the liquid state of matter, but solutions of gases and solids are possible. Common examples of solutions are the sugar in water and salt in water solutions, soda water, etc. A solution is a homogeneous mixture, which means that all parts of the mixture are exactly the same as every other part. Solutions in science as previously learned, solutions are homogeneous, or equal throughout, and contain a solute and solvent.
A solution is a mixture of two or more substances that stays evenly mixed.
As a reminder, the solute is present in less amount than the solvent. If there is no nucleation. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility.


/GettyImages-594836477-58fe53963df78ca159068b1a.jpg)