Why is it important to know the rate law of a reaction? Using the integrated rate law expressions, we can find the concentration of a reaction or product present after sometime in the reaction. The sum of the exponents (a+b+c+…) is the order of the reaction.
Integrated Rate Laws Chemistry Unixpaint
What is the purpose of the integrated rate law?
For example, an integrated rate law is used to determine the length of time a radioactive material must be stored for its radioactivity to decay to a safe level.
The differential rate law can be integrated with time to describe the change in concentration of reactants with respect to time. Rate laws from graphs of concentration versus time (integrated rate laws) in order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs. In general, these ideas are most useful when the rate of the reactions depends only one one chemical species. An integrated rate law comes from an ordinary rate law.
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent.
[a] versus t (linear for a zero order reaction) ln [a] versus t (linear for a 1 st order reaction) Where the exponents, a,b,c,…, may be zero, integers or fractions. If a differential rate law equation is integrated between appropriate limits, the resulting integrated rate law equation shows the dependence of concentration on time. The order of the differential rate equation, of course, determines the form of the integrated equation.
The major difference between the integrated rate law and differential rate law is that the integrated rate law expresses the reaction rate as a function of the initial concentration of one or more reactants after a specific time, whereas the differential rate law expresses the reaction as a function of the change in concentration of one or more reactants during a.
Consider the first order reaction. Kt = 2.303log([r 0]/[r]) (or) k =. We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the. Up to 10% cash back example question #1 :
Rate law is an expression that gives the reaction rate in terms of the concentration of species at any time.
Because using the known rate law , a chemist can work backwards to learn the individual steps and mechanism by which a reaction occurs. They are used to determine the rate constant and the reaction order from experimental data. Based on your data, write the complete rate law, including the value and units for the rate constant. On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed;
The integrated rate law tells you how the concentration of reactant(s) depends on time.
If the initial concentration of the reactant is 0.30 m, how long does it take for the concentration to decrease to 0.15 m? Putting the limits in equation (1) we get the value of c, ⇒ [ a] 0 = c. In this equation, [a] and [b] express the concentrations of a and b, respectively, in units of moles per liter. I.e., the first derivative of concentration with respect to time.
Rate = k [a]a [b]b [c]c.
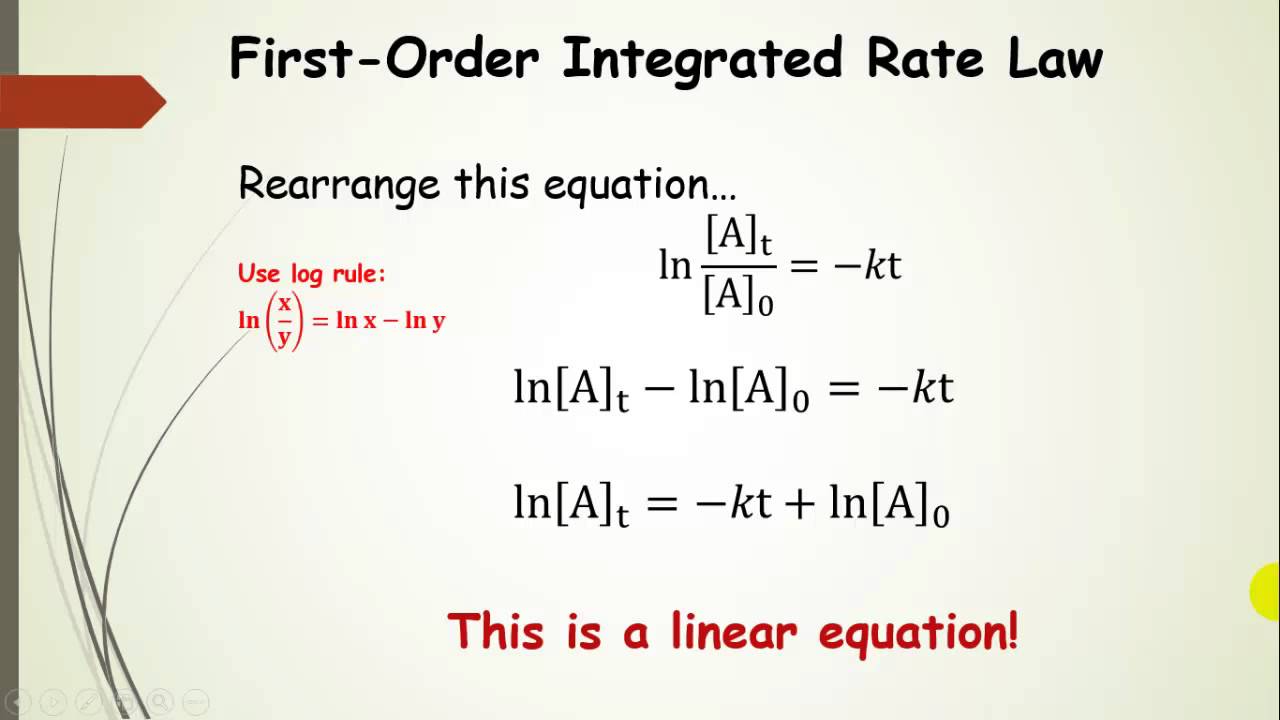
The above equation is known as integrated rate equation for zero order reactions. Ln[ ]=− g p+ln[ ]0 An integrated rate law is an equation that expresses the concentrations of reactants or products as a function of time. An integrated rate law gives the concentration at any time after the start of the reaction.
For several special cases of rate laws, we can integrate the rate law to yield an equation of the concentration of a particular species as a function of time.
Where, [r 0] is the initial concentration of the reactant (when t = 0) [r] is the concentration of the reactant at time ‘t’ k is the rate constant; R =k[a]x[b]y r = k [ a] x [ b] y. For the general reaction aa+bb → c aa + bb → c with no intermediate steps in its reaction mechanism, meaning that it is an elementary reaction, the rate law is given by: It is important to know when such laws apply and in what limits.
For example, an integrated rate law is used to determine the length of time a radioactive material must be stored for its radioactivity to decay to a safe level.
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent. These equations relate reactant concentration with time. Integrated rate laws are derived from rate laws. At time, t=0, [a] = [ a] 0.