The word equation for the reaction between sodium carbonate andhydrochloric acid is:sodium carbonate + hydrochloric acid = sodium chloride + carbondioxide + waterthe balanced equation is: Sodium hydroxide + hydrochloric acid = sodium chloride + water naoh + hcl = nacl + h2o N a o h + h c l → n a c l + h 2 o
9 Aqueous Solutions
What is observed when sodium hydroxide is added to hydrochloric acid and.
Sodium hydroxide + hydrochloric acid → sodium chloride + water.
Can you mix hydrochloric acid and sodium hydroxide? (arrhenius theory) hence it is a neutralisation reaction. When dilute solutions of hydrochloric acid and sodium hydroxide are mixed together, sodium chloride is formed. Hence, the physical state will be aqueous.
Acid + base → salt + water.
Salt and sulfuric acid for the preparation of sodium sulfate in the. How do you balance hydrochloric acid and sodium hydroxide? The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is: Once you know how many of each type.
As naoh gives oh − in water, it is a base.
It also explains how to predict t. Salt and sulfuric acid for the preparation of sodium sulfate in the. Hydrochloric acid and sodium hydroxide equation. Heat (q) developed by the reaction of naoh solution and also hcl solution was 1.255 kj.
Hcl (aq) + naoh (aq) → nacl (aq) + h2o (l) + heat.
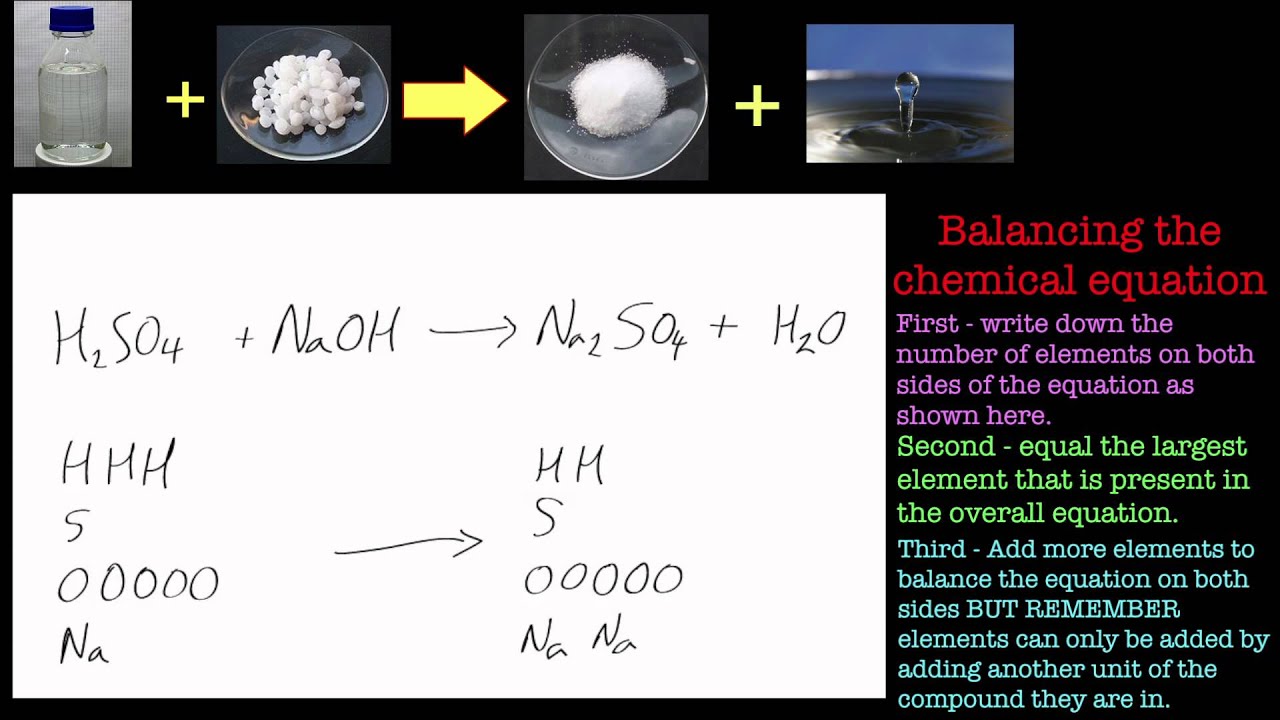
The balanced chemical equation for the reaction of sodium hydroxide with hydrochloric. When strong acids such as h c l (hydrochloric acid) reacts with strong base like n a o h (sodium hydroxide), then neutral salt such as n a c l (sodium chloride) and water are formed. Of atoms of each element in reactants is equal to. We need to combine sulfuric acid and sodium hydroxide to produce sodium sulfate and water.
When sodium hydroxide reacts with hydrochloric.
This chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. Write the neutralization reaction between hydrochloric acid (hci) and sodium hydroxide (naoh), write the equation for this process.? When the excess acid was titrated against sodium hydroxide, 10.9 cm3 of sodium hydroxide solution was. The reaction between sodium hydroxide (naoh) and hydrochloric acid (hcl) is a neutralization reaction which results in the formation of a salt, sodium chloride (nacl), and water (h2o).
It is an exothermic reaction.
Aqueous hydrochloric acid + aqueous sodium hydroxide hydrogen product (water) + sodium product From above we can say that the reaction between hydrochloric acid and sodium hydroxide gives the sodium chloride and water So, net equation will be: To balance naoh + hcl = nacl + h2o you'll need to be sure to count all of atoms on each side of the chemical equation.
Write and balance the equation for the reaction of hydrochloric acid (h2so4) and sodium hydroxide to produce sodium sulfate and water.
What is the balanced equation of sodium hydroxide and hydrochloric acid? The reaction is taking place in water. It is an exothermic reaction. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
The equation is balanced because no.
I understand the acid is the solvent and in combination with naocl produces hypochlorous acid the oxidizing agent. What happens when dilute solution of hydrochloric acid and sodium hydroxide are mixed together? Hydrochloric acid is a strong corrosive acid that is commonly used as a laboratory reagent. The basic formulae hydrochloric acid ishcl and the basic formulae for sodium hydroxide is naoh so the balanced reaction between these two components is.