In seliwanoff’s test (where the sample is heated with acid and resorcinol), aldoses tend to respond at a moderate pace and deliver a slow light pink color. But glucose is an aldose (also called aldohexose) and fructose is a ketose, or a ketohexose. In the first post about carbohydrates, we mentioned that depending on the position of the carbonyl (c1 or c2) the sugar molecule can be an aldehyde or a ketone which are classified as an aldose or a ketose.
Classify The Sugars As Either Aldoses Or Ketoses
‘a’ is for aldehyde in aldose, ‘k’ is for ketone in ketose.
Glucose pyranose & aldose aldehexose.
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. It is the main type of sugar found in our blood and represents our body's primary source of energy. The open chain, the alpha (α) cyclic form, and the beta (β) cyclic form ( fig. Galactose pyranose & aldose aldehexose.
The chemical names of the aldose sugars depend on the number of carbon atoms they possess.
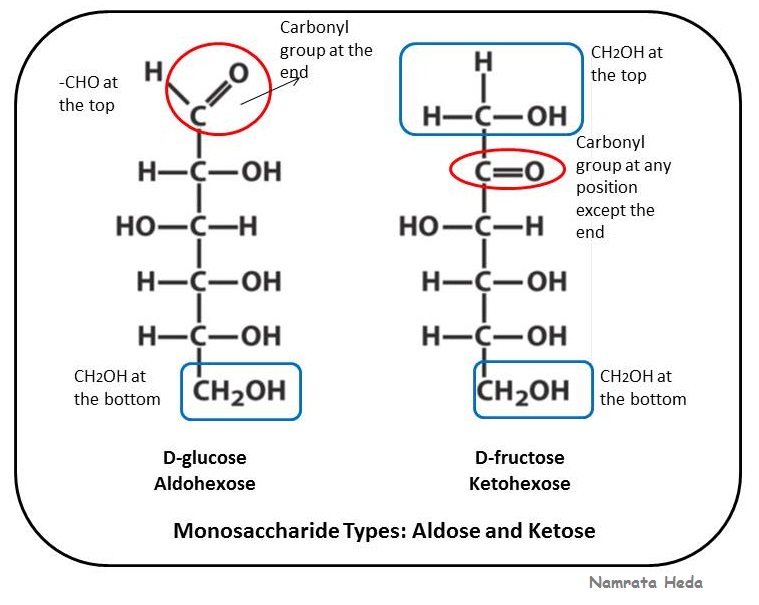
An r group is any molecule or atom that can bind to the carbonyl atom. Since they have no less than one uneven carbon focus, aldoses with at least three carbon particles display stereoisomerism. Aldose gets defined as the monosaccharide that only has one aldehyde group in each molecule and becomes a pure sugar. Aldoses have a carbonyl group (indicated in green) at the end of the carbon chain, and ketoses have a carbonyl group in the middle of the carbon chain.
The general formula for aldoses is c n (h 2 o) n and they start from triose (n=3) structures.
Aldose contains an aldehyde group, and ketose contains a ketone group. What is a reducing sugar? Aldoses such as ribose and glucose can exist in three structural forms: There are no aldose sugars containing formaldehyde (n=1).
Ketose sugars contain ketone functional groups.
The best example of such a structure becomes glycolaldehyde that only has one carbon atom within its structure. Aldose structure has one carbon. Aldoses may decompose into ketose depending on the isomerization reaction. The enzyme is able to act upon a broad range of aldose sugars, encompassing hexoses, pentoses, disaccharides, and trisaccharides, and is able to oxidize glucose to gluconolactone with subsequent hydrolysis to gluconic acid.
The chemical formula of aldose is c n (h 2 o) n.
Both glucose and fructose have the same molecular formula c6h12o6 and are hexoses (6 c). Ketose structure has three carbon atoms. The chemical formula of ketose is written as rcor. Trioses, pentoses, and hexoses have three, five, and six carbon backbones, respectively.
Simple sugars are also subdivided into aldose, a sugar that contains an aldehyde structure, or ketose, a sugar that contains a ketone group.
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. What you need to know about aldose sugar ketose is the monosaccharide that contains ketone group along with the carbon chain. The carbon atoms in the carbon backbone are each bonded to a hydroxyl group. Aldose sugars that contain more than three carbon atoms possess stereoisomerism.
Some of the commonly found aldoses in nature and around us include:
Aldose / ketose number of carbons sugar structure name fructose glucose ribose galactose what's the stereochemistry (chirality) of all biologically relevant monosaccharides? The aldehyde functional group in the organic chemistry stands for the presence of a carbon atom that is single bonded to a hydrogen atom, and is double bonded to an oxygen atom. Mannose pyranose & aldose aldehexose. Another difference is the location of the carbonyl group in each structure.
The most important example is glucose.
A great way to remember this difference is to focus on the first letter in each term: All aldoses exhibit stereoisomerism as they have an asymmetrical carbon center. In what form are monosaccharides biologically, open chain or ring form? The general formula of aldoses is the same as most carbohydrates, cn (h2o)n.
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms.
Aldose is the monosaccharide (carbohydrate molecule) that contains aldehyde group in its structure at the end of carbon chain. Rarely found in its free form, this monosaccharide is a major component of the type of sugar found in. Fructose furanose & ketose ketohexose.