The sum of the exponents (a+b+c+…) is the order of the reaction. Zero [e] t = −kt + [e] 0 [e] vs. Kt = 2.303log([r 0]/[r]) (or) k =.
PPT Unit 15 Chemical PowerPoint Presentation
The unit of k is c o n c e n t r a t i o n u n i t t i m e.
In your flow chart and/or explanation include how you can use plots of section concentration vs.
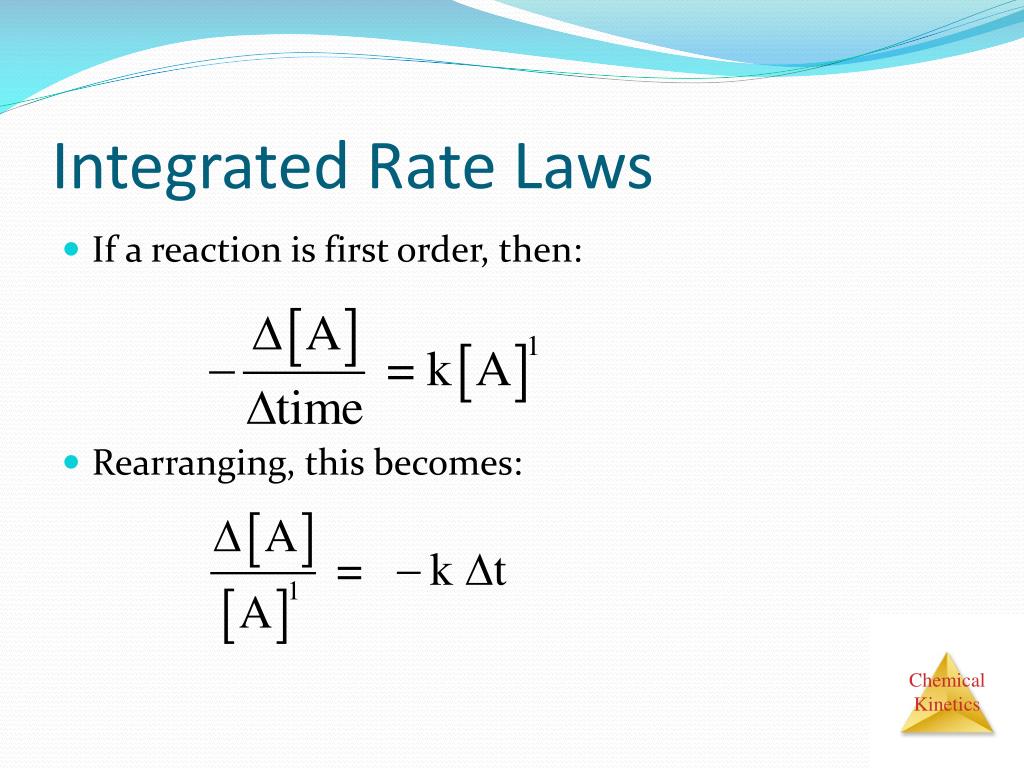
Rate = k [a]a [b]b [c]c. The units are calculated by the following equation: The integrated rate law can be rearranged to a standard linear equation format: The above equation is known as integrated rate equation for zero order reactions.
For a zero order reaction:
Mol l −1 s −1: Putting the limits in equation (1) we get the value of c, ⇒ [ a] 0 = c. These equations can be used to calculate, based on reaction order and the rate constant (which is temperature dependent) integrated rate laws L mol −1 s −1
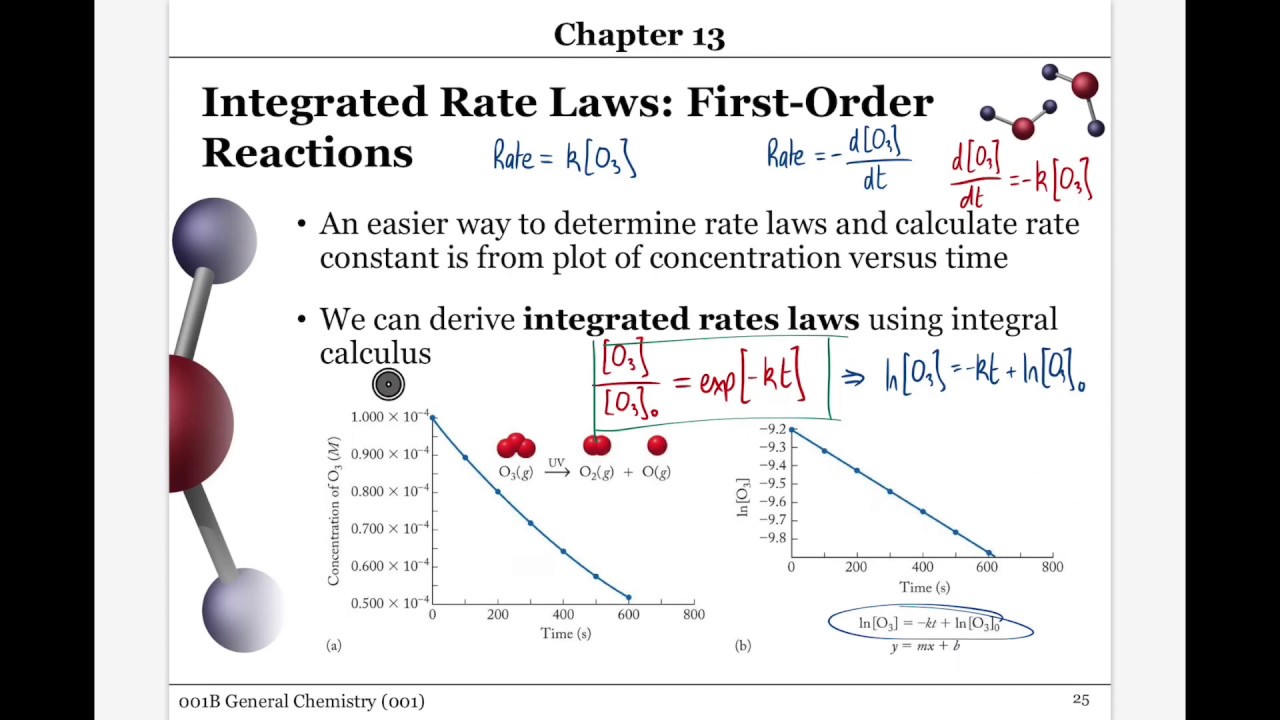
They are used to determine the rate constant and the reaction order from experimental data.
On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed; R a t e = k [ a] n. [a] = − kt + [a]0 y = mx + b. \ (\frac {1} {\left [a\right]}\phantom {\rule {0.1em} {0ex}}=kt+\phantom {\rule {0.2em} {0ex}}\frac {1} { {\left [a\right]}_ {0}}\) where the terms in the equation have their usual meanings as defined earlier.
For the general reaction aa+bb → c aa + bb → c with no intermediate steps in its reaction mechanism, meaning that it is an elementary reaction, the rate law is given by:
K = ( m ⋅ s − 1) × ( m − n) = m ( 1 − n) ⋅ s − 1. Follow this answer to receive notifications. If [ a] = [ b] then, [ a] x + y where x + y = n. It is not necessary that you use any specific unit for concentration or time, but most often t is measured in seconds, and the unit of k is m o l l − 1 s − 1.
The integrated rate law can be rearranged to a standard linear equation format:
This chemistry video tutorial provides a basic introduction into chemical kinetics. Ln[ ]=− g p+ln[ ]0 Rate = k[a]0 = k. R =k[a]x[b]y r = k [ a] x [ b] y.
2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top
The concentration is represented in mol l − 1 or m and time is represented in seconds. Rate = k [a] m/t = k m. It explains how to use the integrated rate laws for a zero order, first. Reaction order integrated rate law characteristic kinetic plot slope of kinetic plot units of rate constant;
Since rate constant is given by rate = k [ a] x [ b] y.
Rate = k [a] 2 rate = k [a] [b] m/t = k m 2. 4 rows k = r a t e [ a] x [ b] y. Annotation for last equation above. Ln[a] (−k)(t) + ln[a]0 y mx + b ln [ a] ( − k) ( t) + ln [ a] 0 y m x + b.
Table 7 summarizes the rate constant units for common reaction orders.
In this equation, [a] and [b] express the concentrations of a and b, respectively, in units of moles per liter. Table 17.1 integrated rate law summary; Where, [r 0] is the initial concentration of the reactant (when t = 0) [r] is the concentration of the reactant at time ‘t’ k is the rate constant; Ln [a] t = −kt + ln [a] 0:
Where the exponents, a,b,c,…, may be zero, integers or fractions.
So, k = r a t e [ a] x [ b] y. Answered may 4, 2015 at 3:28.