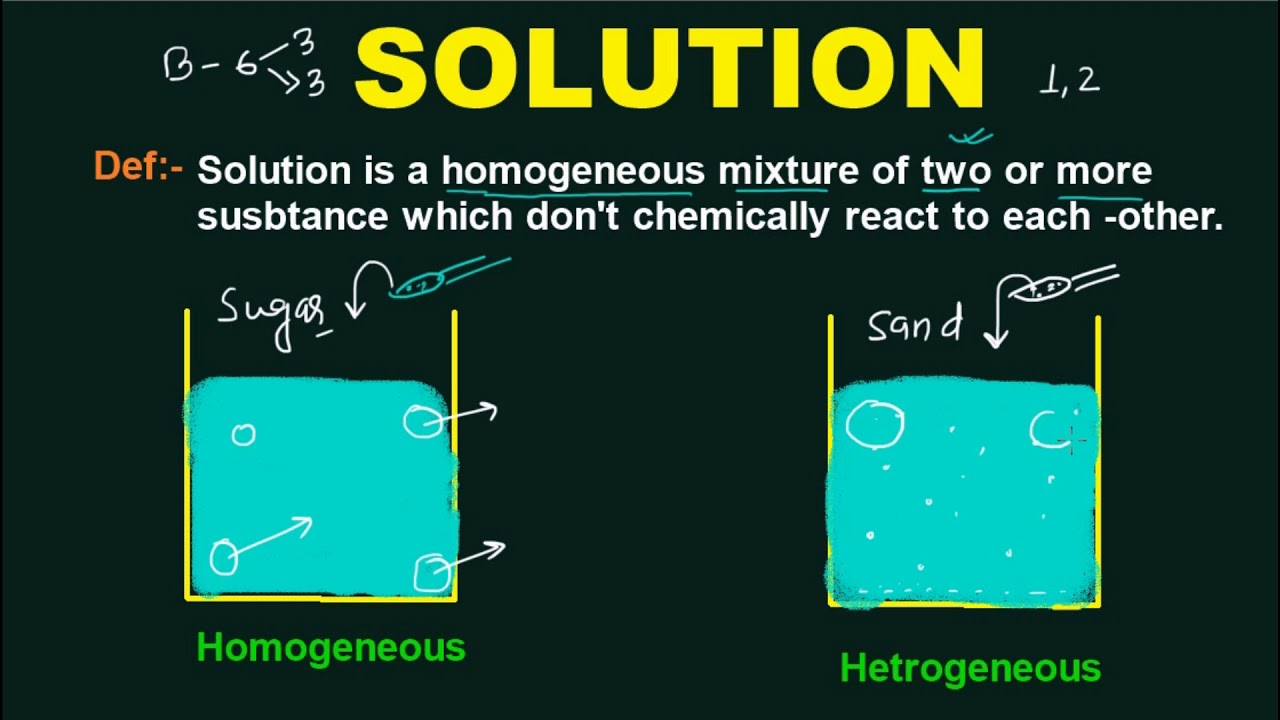
A solution to a problem or difficult situation is a way of dealing with it so that the difficulty is removed. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. A solution is a homogeneous type of mixture of two or more substances.
Solution Definition Magnifier Showing Achievement Vision
Note that the solvent is the substance that is present in the greatest amount.
Solutions with the stable concentration of hydrogen ions and thus typically with no change in ph which is almost independent of dilution and which change very little with small additions of a strong acid or alkali are called buffers.
A set of values of the variables that satisfies an equation. A solution has two parts: The answer to a problem: There is particle homogeneity i.e.
1.a homogeneous mixture of one or more substances (solutes) dispersed molecularly in a sufficient quantity of dissolving medium (solvent).
Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. If you find an answer to a question, both the answer and how you got there is the solution. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. [countable] a way of solving a problem or dealing with a difficult situation synonym answer.
The substance that dissolves in a solvent to produce a homogeneous mixture.
Answer, result… find the right word. When we put 5 in place of x we get: The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle. An action or process of solving a problem.
5 + 2 = 7 is true, so x = 5 is a solution.
X + 2 = 7. A solution consists of a solute and a solvent. It can also be described in simple terms as a solution that prevents any ph change. A solution is a liquid or solid which is made by dissolving a solid, liquid, or gas in the pure liquid or solid.
The substance in which a solute dissolves to produce a homogeneous mixture.
A value, or values, we can put in place of a variable (such as x) that makes the equation true. A solvent is a substance that dissolves another substance by pulling the molecules apart through electrochemical interactions. A solute and a solvent. The term solution is commonly applied to the liquid state of matter, but solutions of gases and solids are possible.
A solution is a homogeneous mixture of one or more solutes dissolved in a solvent.
A solution has two parts: A solution which does not dissolve any. (image to be added soon) you must have seen many types of solutions in general such as soda water, sharbat, salt solution etc. The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle.
Something attained by mental effort and especially by computation.
For names of specific solutions, see under the name. The solute is the substance that is dissolved in the solvent. 1 a means of solving a problem or dealing with a difficult situation. Technically speaking, a solution consists of a mixture of one or more solutes dissolved in a solvent.
A solute and a solvent.
A solution is all about solving or dissolving. If you dissolve a solid into a liquid, you've created a different kind of solution. A solution may exist in any phase. In a solution, all the components appear as a single phase.
The amount of solute that.
To accurately be called a solution, therefore, the antivirus software should be bundled with related products, such as a spam filter or backup service. A solution is a homogeneous mixture of two or more substances. A liquid into which a solid has been mixed and has dissolved: ‘there are no easy solutions to financial and marital problems’.
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm.
Technically speaking, a solution consists of a mixture of one or more solutes dissolved in a solvent. In a stricter sense, however, an it solution is an aggregation of products and services, as opposed to a single, discrete product. Common examples of solutions are the sugar in water and salt in water solutions, soda water, etc. 2.in pharmacology, a liquid preparation of one or more soluble chemical substances, which are usually dissolved in water.
Homogeneous means that the components of the mixture form a single phase.
(səluːʃən ) explore 'solution' in the dictionary. An answer to a problem : Attempts to find a solution have failed. Air, for example, is a solution consisting chiefly of oxygen and nitrogen with trace amounts of.
A solution is a homogeneous mixture of solvent and solute molecules.