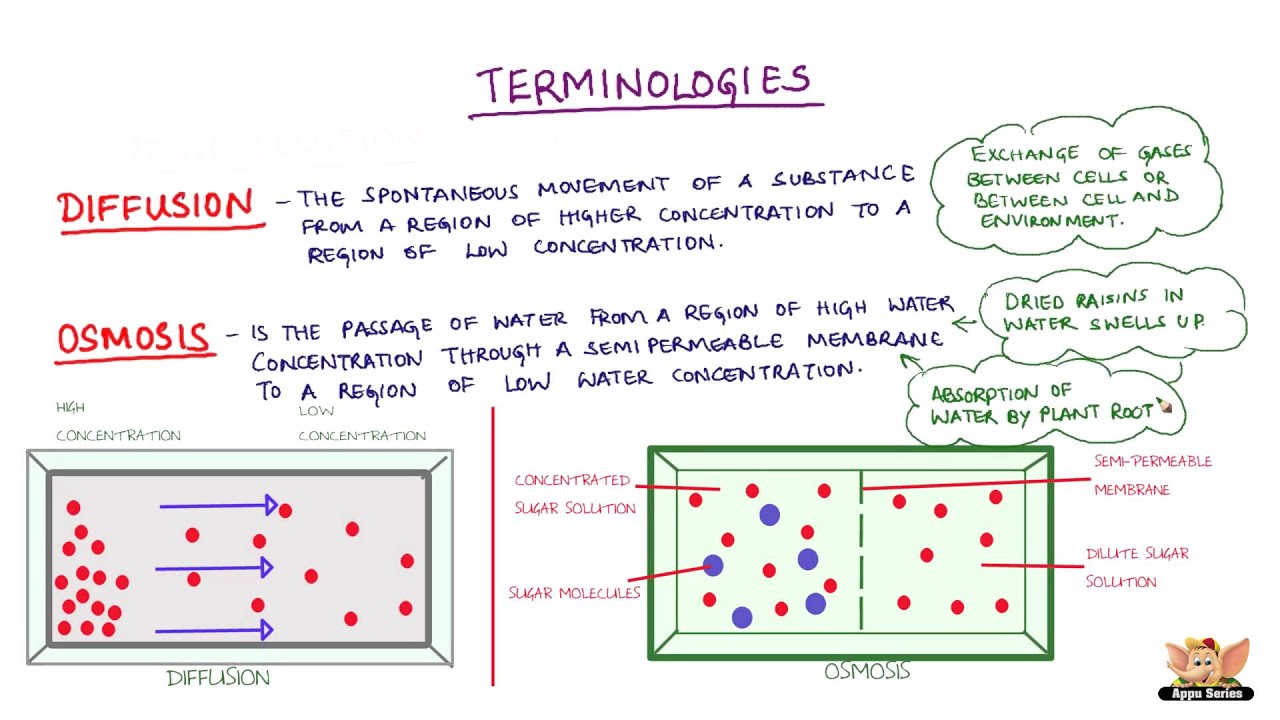
In chemistry, we call a solution isotonic. A solution is a homogeneous mixture of two or more substances. A method or process of dealing with a problem:
Osmosis Simple Definition Biology WCLN Osmosis water
A solution consists of a solute and a solvent.
Hypertonic solutions have high osmotic pressure.
A hypotonic solution is a solution that has a lower solute concentration compared to another solution. A hypertonic solution contains a higher concentration of solutes compared to another solution. An isotonic solution is one that has the same osmolarity, or solute concentration, as another solution. The opposite solution with a lower concentration is known as the hypotonic solution.
The amount of solute that can be dissolved in solvent is called its solubility.for example, in a saline solution, salt is the solute dissolved in water as the solvent.
A solute and a solvent. If a cell is placed in a hypertonic solution, the cell is considered hypotonic. A solution typically consists of the dissolved material called the solute and the dissolving agent called the solvent. The answer to a problem or the explanation for.
A solution of 5%sugar and 0.45% salt.
In other words, a buffer is an aqueous solution of either a weak acid and its conjugate base or a weak base and its conjugate acid. To avoid confusion with a mixture, which is heterogeneous (multiple compounds exist in different structures), solutions are homogeneous, meaning that the solute’s atoms are uniformly distributed in the solvent (ex. Hypotonic is a description of the solute content of one solution in relation to another solution. The effect is zero water flow between the two solutions, although water is moving both ways.
Hypotonic solutions have low osmotic pressure.
Thus, a solution is a mixture containing two components (solute and solvent). A type of homogenous mixture in which the particle s of one or more substance s (the solute) are distributed uniformly throughout another substance (the solvent ). An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water. The amount of solute that.
A solute and a solvent.
We use this term for describing solutions, chemistry, and muscles a few times in human biology. When two solutions are in contact, solute or solvent moves until the solutions reach. Scientists must describe cell contents compared to the environment. A plant cell becomes turgid when putting in a hypotonic solution.
A solution has two parts:
The substance in which a solute is dissolved to form a solution. We use these solutions commonly as intravenously infused fluids in the patients admitted in the hospitals. A homogeneous mixture is a type of mixture with. A solution is a homogeneous mixture with one or more solute particles in the solvent phase (either gas, liquid or solid).
A solution is a mixture of two or more substances that have been dissolved in a liquid medium.
A solution whose concentration is less than the cell sap or inside a cell. If these two solutions are separated by a semipermeable membrane, water will flow in equal parts out of each solution and into the other. A solution has two parts: Firstly, isotonic is a kind of solution that consists of the same salt concentration as the cells and the blood.
Buffers are used to maintain a stable ph in a solution, as they can neutralize small quantities of additional acid of base.
It is a type of solution which has a lower solute concentration than another solution. A hypertonic solution is one which has a higher solute concentration than another solution. Sodium chloride 0.9% is considered to be approximately isotonic with. A solution with an osmotic pressure equal to that on the other side of a semipermeable membrane.
A solution may exist in any phase.
A solution is a mixture of two or more substances, often in liquid form. When we talk about solutions, we can compare two solutions that are separated by a semipermeable membrane. Sought a solution to falling enrollments. The solute is the substance that is dissolved in the solvent.
© 2003 by saunders, an imprint of elsevier, inc.
Solution (chemistry) synonyms, solution (chemistry) pronunciation, solution (chemistry) translation, english dictionary definition of solution (chemistry). A solution consists of a solute and a solvent.the solute is the substance that is dissolved in the solvent. A solution cannot be hypotonic, isotonic or hypertonic without a solution for comparison. It is a type of solution which has a greater concentration of solute than another solution.
A buffer may also be called a ph buffer, hydrogen ion buffer, or buffer solution.
A solution is a homogeneous type of mixture of two or more substances. To learn more about properties, types, videos &. The substance that is dissolved in a liquid (solvent) to form a solution. A mixture of water and nondissolved materials.
It is used in biology to help scientist describe cells.
Measurement system used to indicate the concentration of hydrogen ions (h+) in solution that ranges from 0 (most acidic) to 14 (most basic) acid.