The reaction of butadiene gas (c 4 h 6) with itself produces c 8 h 12 gas as follows: R a t e = k [ ( c h 3) 3 c b r] the rate of the reaction can also be written as. [ a ] 0 = 0.200 mol/l, k = 5.76 × × 10 −2 l/mol/min, and t = 10.0 min.
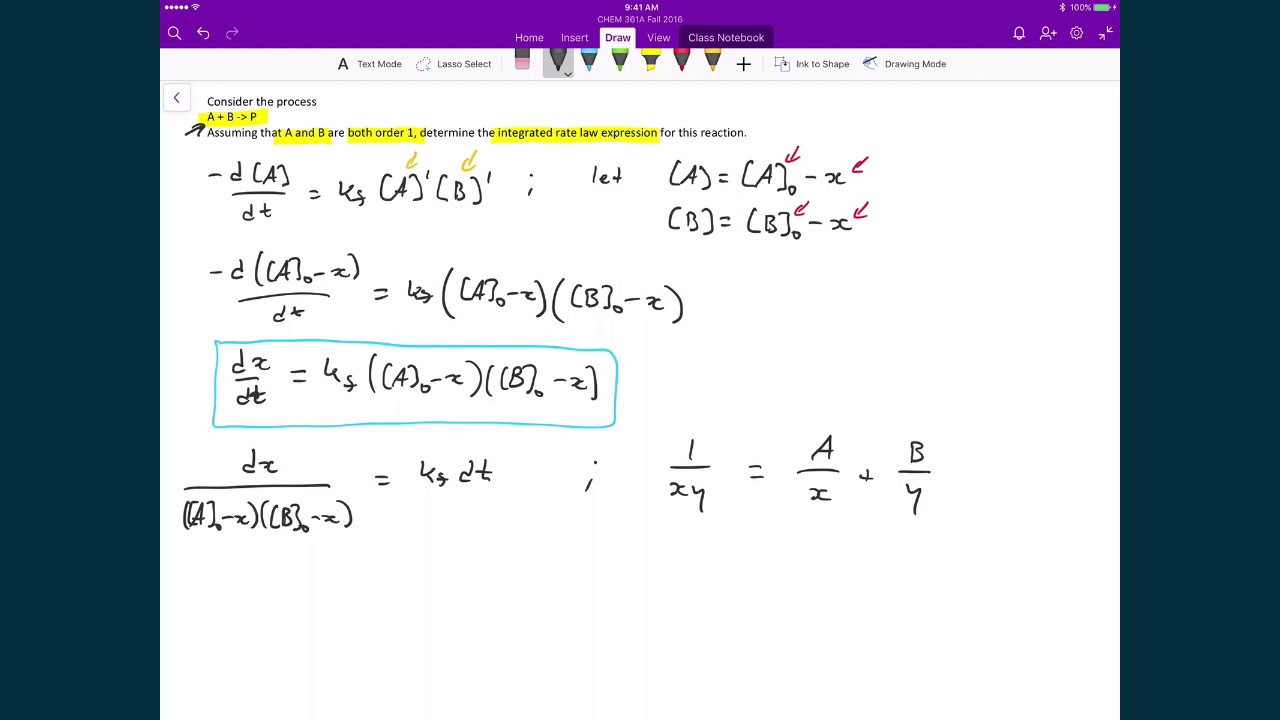
Derive the integrated rate law of the second order
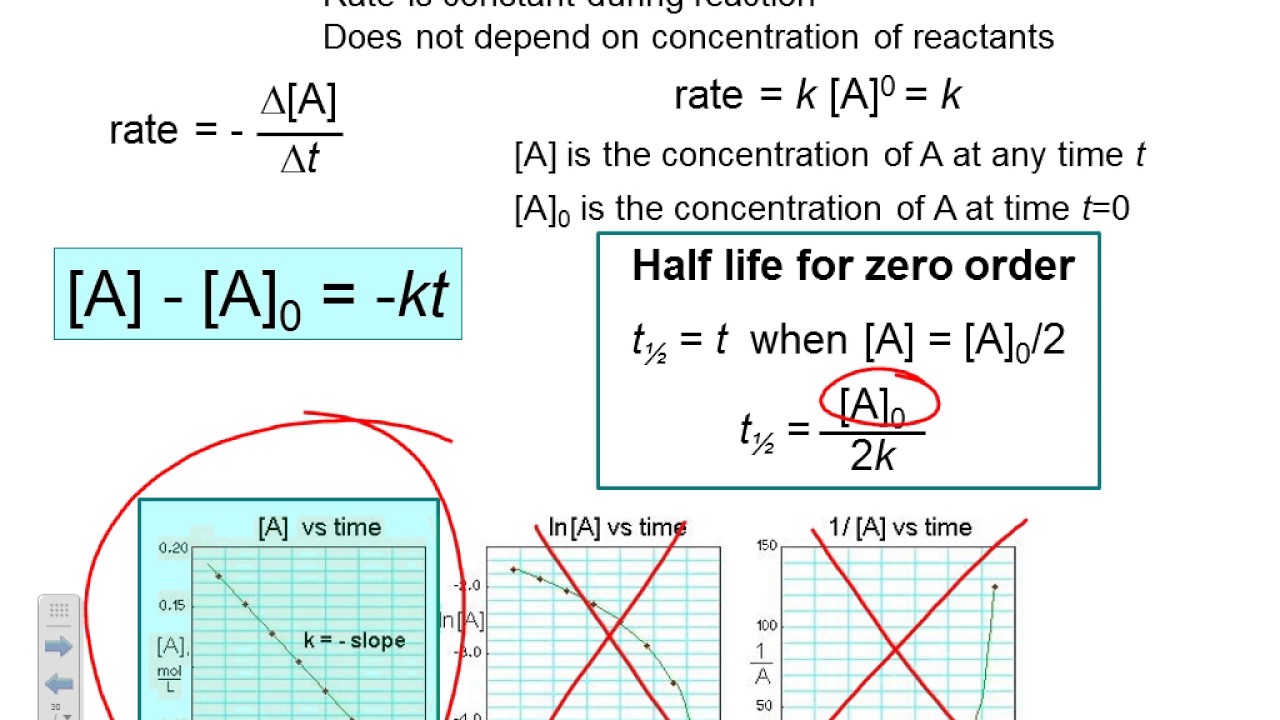
Integrated rate laws can be used to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent.
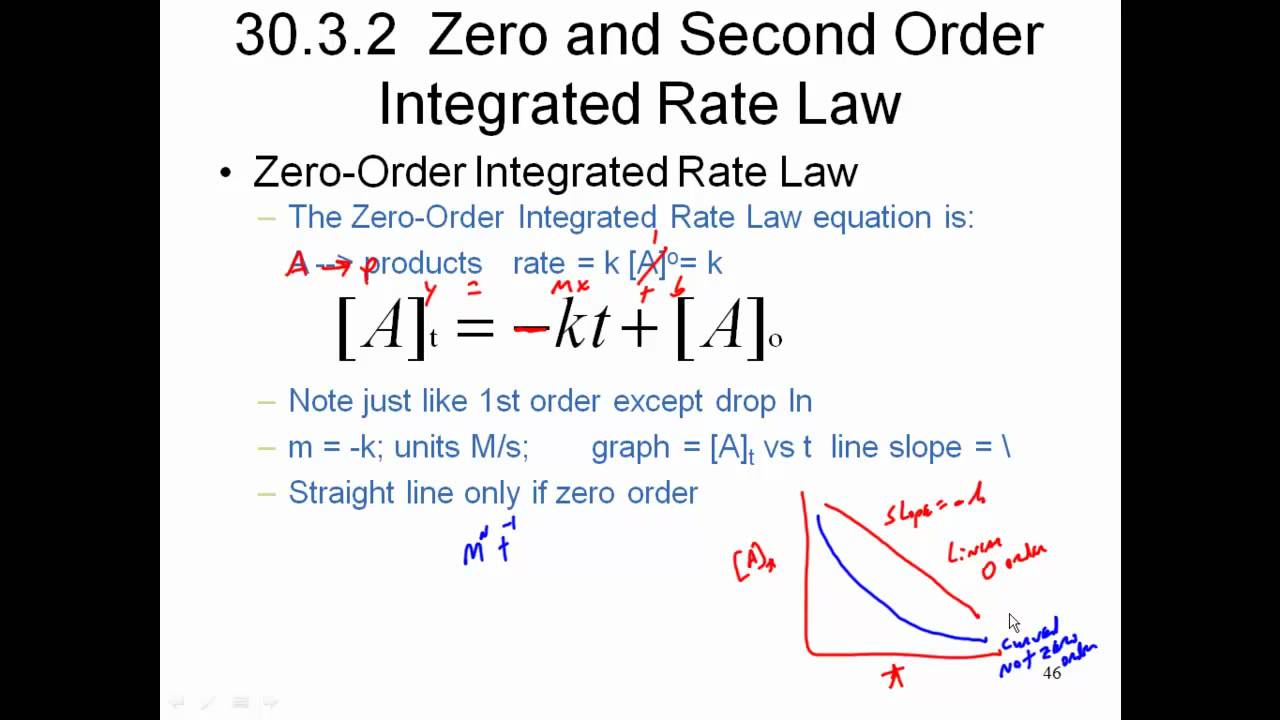
The equation for the second order integrated rate law takes the form y = mx +b, where y = 1/a;
Since rate = k[a] 2. When a reaction is of second order with regard to a specific reactant, an increase in its quantity causes the rate to grow. For second order reaction let [a] = a then rate = ka 2 (i) if [a] = 2a then rate = k (2a) 2 = 4 ka 2. ∴ the rate of reaction will be 1/4 th.
This chemistry video tutorial provides a basic introduction into chemical kinetics.
1 [a]t = kt+ 1 [a]0 y = mx+b 1 [ a] t = k t + 1 [ a] 0 y = m x + b. It explains how to use the integrated rate laws for a zero order, first. Determine the concentration of hi after 90 days if the initial concentration of c. Differential rate law for one pair of observed rate and concentration values, because experimental error is more likely to be averaged out.
The mechanisms of chemical reactions.
For example, an integrated rate law helps determine the length of time a radioactive material must be stored for its radioactivity to decay to a safe level. \[\ce{2c4h6}(g) \ce{c8h12(g)} \] the reaction is second order with a rate constant equal to 5.76 × 10 −2 l/mol/min under certain conditions. The second order integrated rate law is {eq}1/[a]_t = kt + 1/[a]_0 {/eq}. 1 [𝐴𝐴 ] = (6.4 −× 10.
That means the rate law is.
K is the rate constant; Rate = k[a] rate = k[a] 2: ( c h 3) 3 c b r + o h − → ( c h 3) 3 c o h + b r −. Thus, the graph of the second order integrated rate law is a straight.