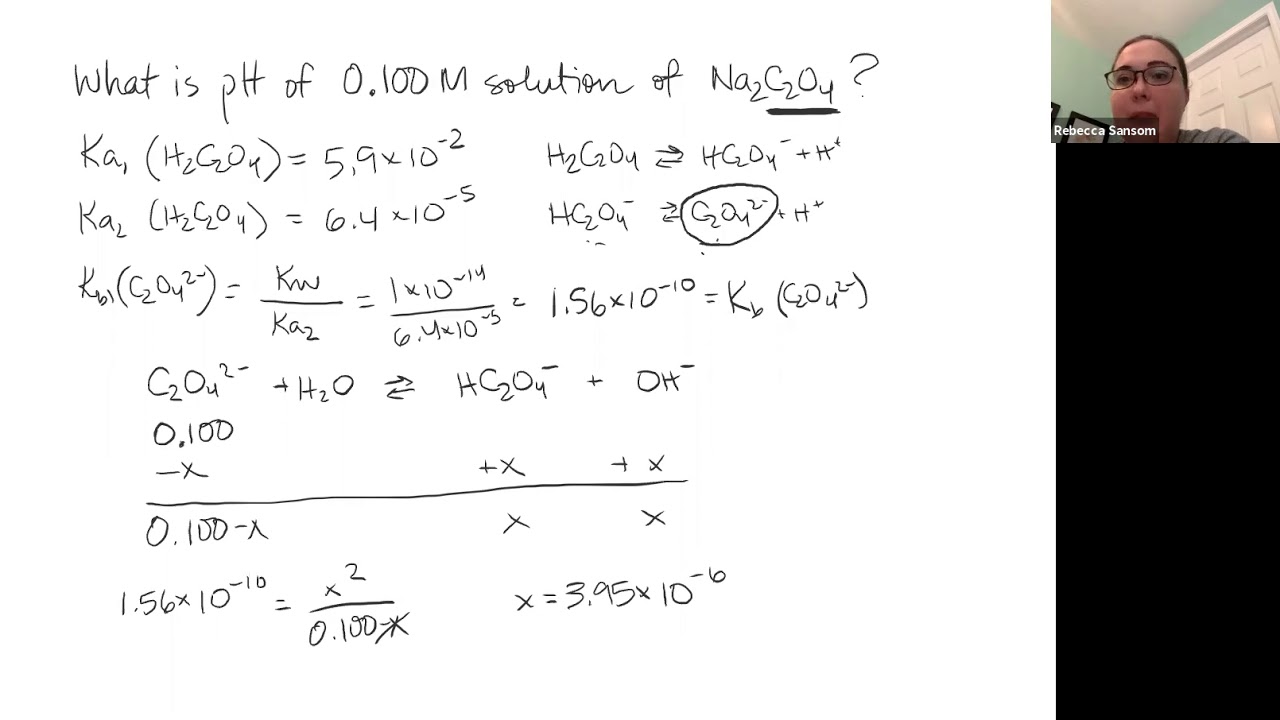First, write the equation for the dissolving process, and examine eachion formed to determine whether the salt is an acidic, basic, or neutralsalt. When dissolved in water, they dissociate into individual hydrated ions that may or may not affect the ph of the solution. The aqueous solutions of these salts are neutral with ph value of 7.
pH of a Salt Solution YouTube
[kb (ch3nh2) = 4.38 × 10 −4] this salt contains ch3nh3 + and cl−.
Determine the ph of the solution.
They hydrolyze to form a basic solution. Refer to the observation table 2. 1 salts that are from strong bases and strong acids do not hydrolyze. List the known values and plan the problem.
Neutral electrolyte:a salt which does not produce hydronium ions (h3o.
As you likely know, sodium fluoride (naf) is an ionic compound. 8.3 acid/base properties of salt solutions. The ph of salt solutions prelab questions fill in the following table about salt solutions solution parent acid strength parent base strength acidic or basic nh 4 c 2 h 3 o 2 nacl nac 2 h 3 o 2 nh 4 cl nahco 3 na 2 co 3 1) write the equation for the reaction of ammonium ion with water 2) write the equation for the reaction of bicarbonate ion with water When you consider the ph of salt solutions, one important concept in understanding this is lechatelier's principle.
The ph of the resulting solution can be determined if the k b of the fluoride ion is known.
View the ph of salt solutions.docx from chem sch4u at brookfield high school, ottawa. There are several guiding principles that summarize the outcome: The solution will be acidic. 3 salts of weak bases and strong acids do hydrolyze, which gives it a ph less than 7.
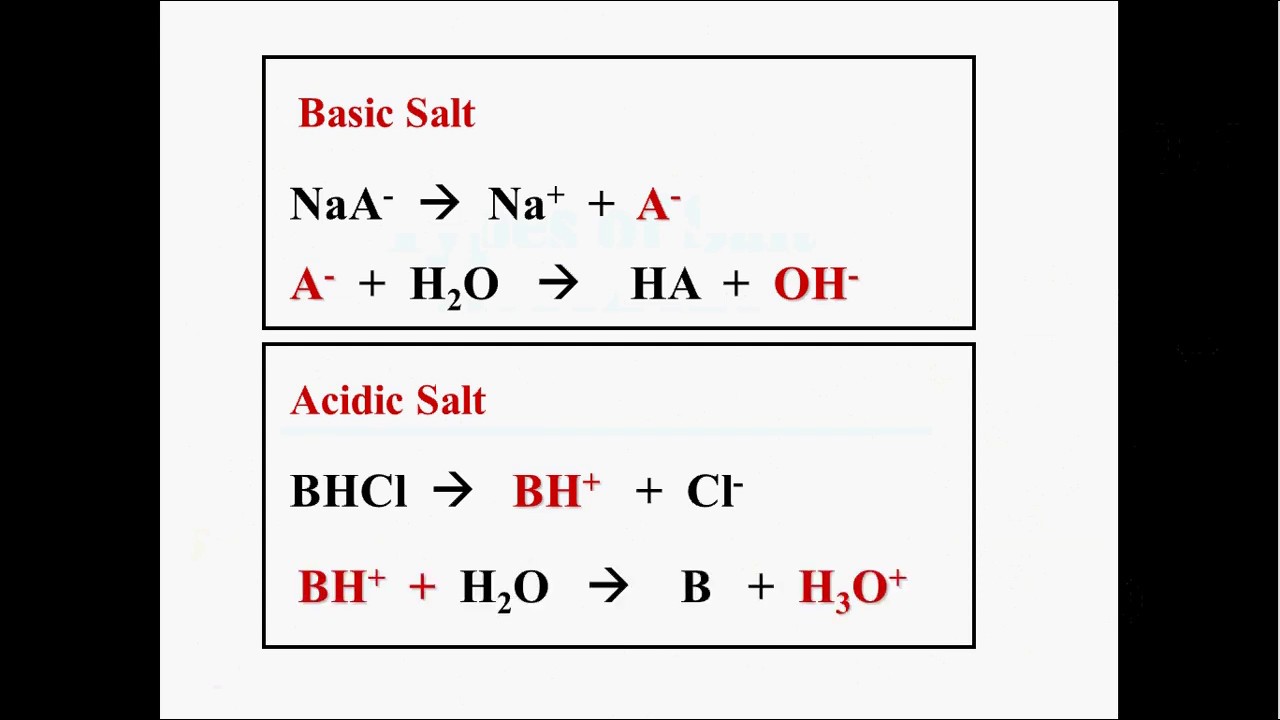
Acidic salts (ph < 7) are conjugate acids of weak bases
To further elaborate, let's consider the 1.0 m solution of naf, found in the first row of table 3. Cl− is the conjugate base of a strong acid, hcl, and therefore has no base strength in water. It is a measure of how acidic or basic a. It is a compound obtained when an acid and base react and this salt gets anion from acid and cation from the base and this process is called neutralization.
The ph of a salt solution is determined by its component anions and cations.
Therefore, ch3nh3 + is a weak acid in water. Ph of salt solutions 2. Calculate the ph of the solution. 2 salts that are from strong bases and weak acids do hydrolyze, which gives it a ph greater than 7.
Ch3nh3 + is the conjugate acid of the weak base, ch3nh2.
20.0 g of sodium fluoride is dissolved in enough water to make 500.0 ml of solution. Sodium chloride (nacl), potassium nitrate (kno 3 ), sodium sulphate (na 2 so 4 ), etc., are salts of this category. Acid + basic = salt + water. What is the ph of a salt solution.
It is because when hydrolysis of basic salt takes place, the conjugate base of the weak acid is formed in the solution.
When mixed with water, sodium fluoride dissociates into its ions. Up to 24% cash back 1. Calculate the ph of a 0.10 m solution of ch3nh3cl. (i) salts of strong acids and strong bases :
Yes, the evidence supports my predictions of all chemicals except
The aqueous solutions of all kinds of salts do not have the same ph value. The k b of the fluoride ion is 1.4 × 10 − 11. Since x represents the hydroxide ion concentration, we can convert it into poh and than find the ph. To calculate the ph of a salt solution, you need to write the equation for the interaction with water and determine whether the salt is derived from a strong/weak acid or base.
Calculate the ph of a 0.500 m solution of kcn.
Ph is less than 7. For example, in ammonium nitrate (nh 4 no 3 ) solution, no 3 − ions do not react with water whereas nh 4 + ions produce the hydronium ions resulting in the acidic solution. 3 rows calculate ph of solution: