Pka of acetic acid = 4.76. Made by faculty at the university o. A buffer solution was made by dissolving 10.0 grams of sodium.
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 1 YouTube
Acidic buffers are solutions that have a ph below 7 and contain a weak acid and one of its salts.
What are buffer solution give an example for acidic buffer?
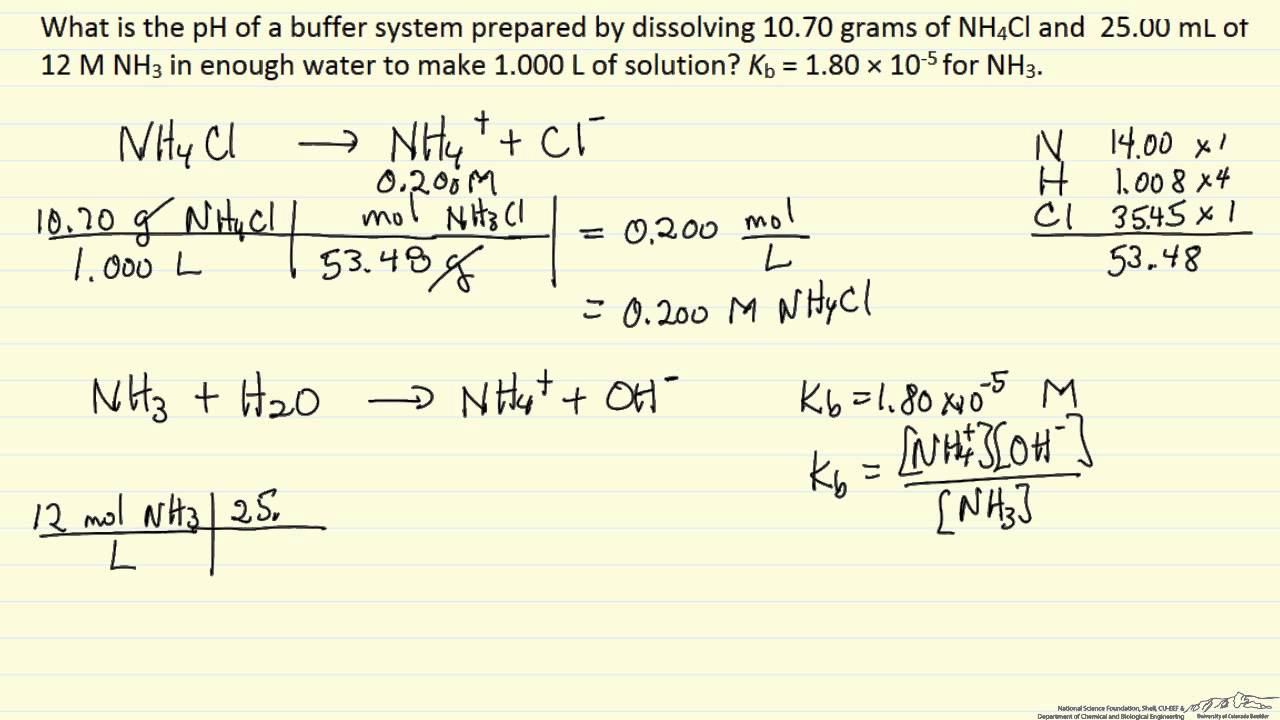
(iii) bicarbonate/carbon dioxide (carbonic acid) (iv) dihydrogen phosphate/biphosphate. The k a of the acid also needs to be known. In order to calculate the ph of the buffer solution you need to know the amount of acid and the amount of the conjugate base combined to make the solution. [latex]nh_4^+ \rightleftharpoons h^+ + nh_3[/latex]
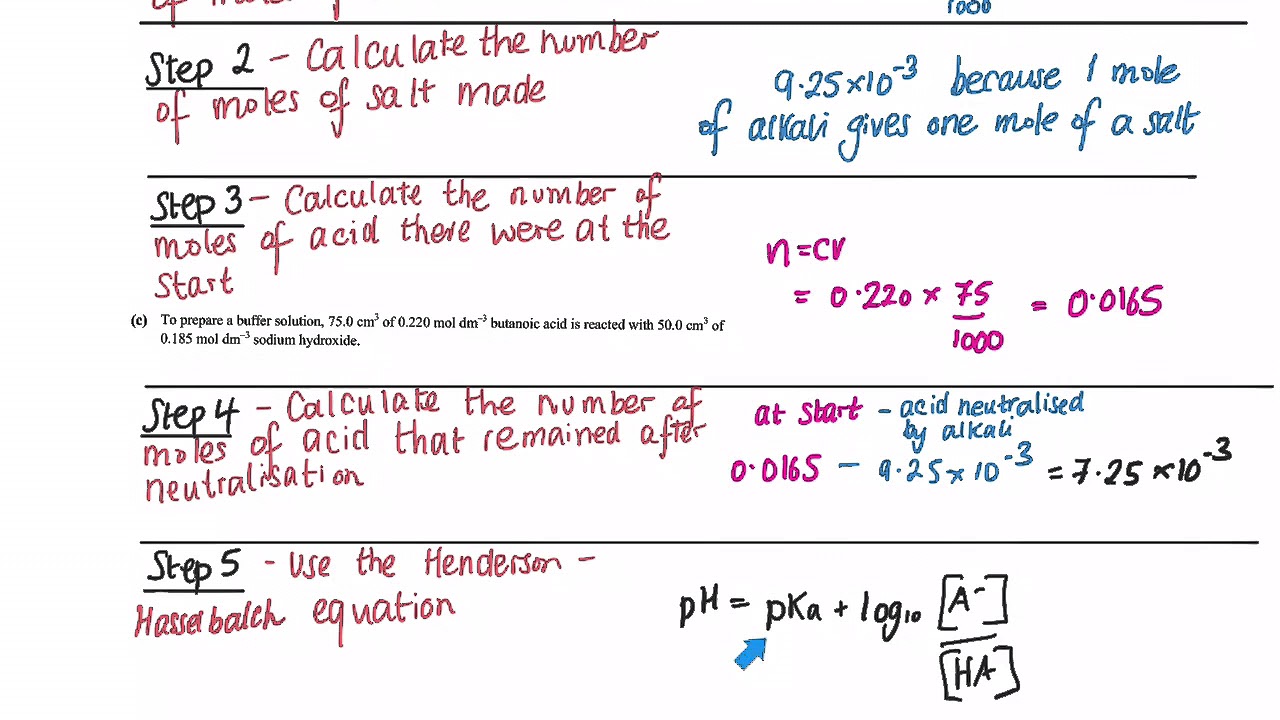
Calculate the volume of 0.2m solution of acetic acid that needs to be added to 100 ml of 0.2m solution of sodium acetate to obtain a buffer solution of ph 5.00.
Ph = pka + log( [a−. From the calculation above, the ph of buffer solution is 7.38. Therefore, to make a buffer solution of a particular ph, we used a weak acid whose pka is close to the desired ph value. Buffer solutions do not have a definite ph.
Is acetic acid a strong acid?
Following components are given in the question: Blood in our body is a good example of a basic buffer. The ph of a solution, if it is equal to 7 is considered neutral; The ph of a buffer solution changes slightly on the addition of a small amount of acid or base.
Many people experience severe anxiety and suffer from alkalosis.
Calculate the ph of a buffer solution made from 0.20 mol/l hc₂h₃o₂ and 0.50 mol/l c₂h₃o₂⁻. The \({\rm{ph}}\) of blood in the human body is \(7.4.\) both higher and lower \({\rm{ph}}\) values of blood may be fatal. They contain a weak base and a salt of the weak base. If the concentration of the salt and the acid in the above relation is the same, we have ph = pka.
An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride (nh 3 (aq) + nh 4.
Calculate the ph of a buffer solution consisting of 0.051 m nh 3 and 0.037 m nh 4 +. The equation for the reaction is as follows: Solved example for you the ph of a buffer solution does not change on dilution. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added.
A buffer solution is composed of a weak acid, and its conjugate base in appreciable concentrations.and so five examples are.
Calculation of the ph of a buffer solution. The ph of a solution, if greater than 7 is considered basic. Calculate the ph of a buffer solution that initially consists of 0.0500 m nh 3 and 0.0350 m nh 4 +. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt.
Therefore this buffer solution is very beneficial for life.
The aqueous solution of an equal concentration of ammonium hydroxide and ammonium chloride has a ph of 9.25. First you need to rearrange the equation accordingly. Calculate the ph of an unbuffered 0.010m acetic acid solution. The ph of a solution, if is less than 7 is considered acidic;
The ratio of acetate to acetic acid required to get a ph of 5.20 is 2.75.
To calculate the ph of a buffer solution made up of a weak acid and its conjugate salt, we can apply the henderson equation, ph = pka + log[salt]/[acid]. Human blood has a natural ph of 7.4. These values can then be substituted into the k a expression to calculate the concentration of h 3 o + as shown in the following example. An example of an alkaline buffer solution is a mixture of ammonium hydroxide and ammonium chloride (ph = 9.25).
For example, a mixture of acetic acid and sodium acetate acts as a buffer solution with a ph of about 4.75.
The ph of buffer solution does not change on standing for long. Consider, for example, the following problem: Determine the ph of a solution prepared by dissolving 0.35 mole of ammonium chloride in 1.0 l of 0.25 m aqueous ammonia. \({\rm{ph}}\) plays a vital role in the biological system;
Ph of the buffer = 5.20.
Examples of a few acidic solutions are vinegar or lemon juice whereas examples of some basic solutions are milk. The acid dissociation constant of hc₂h₃o₂ is 1.8 × 10⁻⁵. These amounts should be either in moles or in molarities. 1) this is a buffer solution, with a weak base (the ammonia) and the salt of the weak base (the ammonium chloride) in solution at the same time.
Buffer solutions are prepared to maintain the ph of a solution.
Pka of acetic acid is 4.74. Ha + h₂o → h₃o⁺ + a⁻. The ph of these solutions is above seven. In this article we will discuss the definition of a buffer solution, the buffer system in the body, the formula and calculation of the ph in the buffer solution, and examples.
Alkalosis is a disease in which blood ph is excessively high.