It explains the concept, compo. In chemistry, ph is a measure of the hydrogen ion (h +) concentration in a solution. [ca] = concentration of the conjugate acid;
CM4106 Review of Lesson 3 (Part 1)
Unlike project gutenberg, which gives all books equal billing, books on amazon cheap reads are organized by rating to help the cream rise to the surface.
Ph of a buffer (henderson equation) calculator.
For solutions of a weak bases sometimes it is. Ha⇌ h++a− h a ⇌ h + + a −. Download free equation to calculate ph of a buffer solution equation to calculate ph of a buffer solution | 65b9f6d6726f6fb878b2b29682334293 general organic and. With bases, if the value of an equilibrium constant is known in the form of a base association constant, k b the dissociation constant of the conjugate acid may be calculated from
Calculation of the ph of a buffer solution after addition of a small amount of strong acid
The strength of a weak acid is usually represented as an equilibrium constant. Thus it can be safely used in the case of phosphoric buffers but not in the case of citric acid buffers. A basic solution will have a ph above 7.0, while an acidic solution will have a ph below 7.0. Buffer capacity is the capacity of a buffer solution to resist change in its ph.
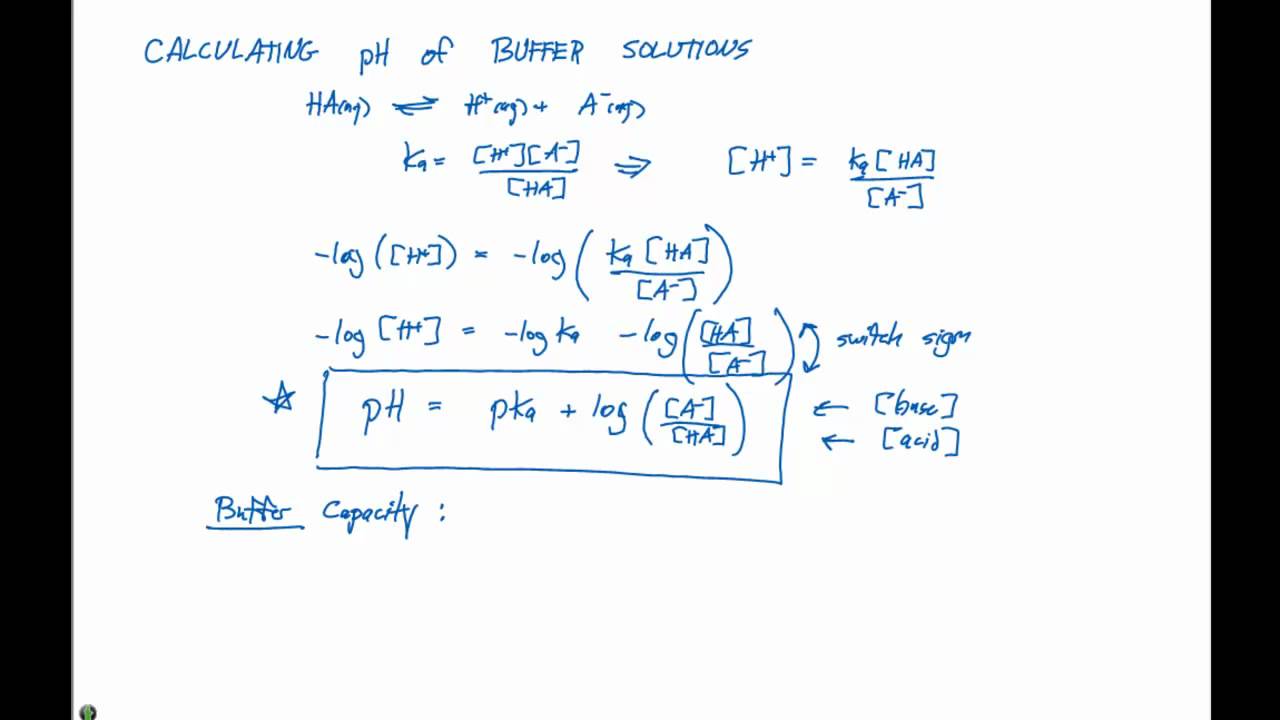
Ha + h 2 o ⇋ h + + a −.
This video will look at how you can use ka to work out the ph of a buffer solution. Ph = 4.7 + log(0.6m /0.4m) = 4.7. Taking negative logarithms of both sides, we obtain. Therefore, the ph of the buffer solution is 7.38.
The equation is given by, ph = pka + log [salt] / [acid] the ph of any acidic buffer solution is always less than 7 and the ph of any basic buffer solution is always greater than 7.
[cb] = concentration of the conjugate base ; Do all buffers have a ph of 7? If we substitute the values in equation 1 above, we will get: The balanced equation for a buffer is:
Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its ph is.
A buffer is a solution which can resist the change in ph. This equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its salt in a buffered solution. This answer is the same one we got using the acid dissociation constant expression. Calculate the ph of a buffer solution that initially consists of 0.0500 m nh 3 and 0.0350 m nh 4 +.
[h+] = hydrogen ion concentration.
In the course of guides you could enjoy now is equation to calculate ph of a buffer solution below. [latex]\text{nh}_4^+ \rightleftharpoons \text{h}^+ + \text{nh}_3[/latex] we know that initially there is 0.0350 m nh 4 + and 0.0500 m nh 3. The equation for the reaction is as follows: A ph of 7 is neutral, a ph less than 7.
Calculating ph of buffer solutions:
Ka= acid dissociation constant ; Ph = pka + log [a−] [ha] ph = p k a + log [ a −] [ ha] where p ka is the negative of the common logarithm of the ionization constant of the weak acid (p ka = −log ka ). Taking, negative log of rhs and lhs: By knowing the k a of the acid, the amount of acid, and the amount of conjugate base, the ph of the buffer system can be calculated.
The ph of a buffer can be calculated from the concentrations of the various components of the reaction.
Substituting the values, we get: Buffer solution ph computer simulation | chemdemos a useful calculation that is a must know for the exam!