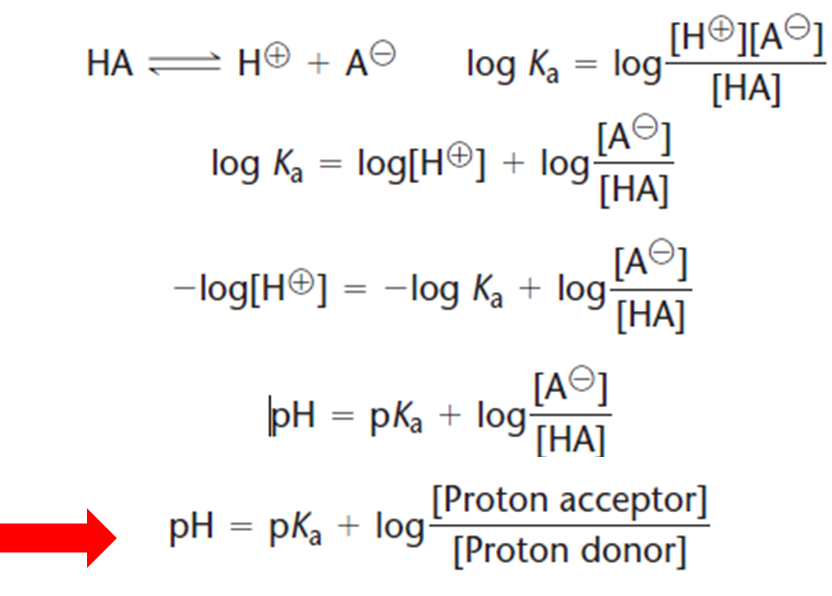
The buffer capacity is optimal when the ratio is 1:1; The above equation for k a can be rearranged to solve for the hydronium ion concentration. We review their content and use your feedback to keep the quality high.
Acids And Bases Ph Scale Worksheet / Acids, Bases, & pH
A mixture of acetic acid and sodium acetate can act as a buffer solution.
The ph of a buffer depends not on the concentration of the buffer ions but on a thermodynamic quantity called the activity coefficient.
Buffer solutions are used in fermentation , food preservatives, drug delivery, electroplating, printing, the activity of enzymes, blood oxygen carrying capacity need specific hydrogen ion concentration. Also know, what factors influence the effectiveness of a buffer? Suppose you wish to prepare a buffer solution to keep the ph at 4.30. 1.the ph of the buffer solution depends upon the concentration of.
From the calculation above, the ph of buffer solution is 7.38.
Ratio of the salt to the acid or base. Assume no change in solution volume. Now, if we know the value for k a, we can calculate the hydrogen ion concentration and therefore the ph. By knowing the k a of the acid, the amount of acid, and the amount of conjugate base, the ph of the buffer system can be calculated.
The ph can be kept constant with the help of?
Ph of any buffer depends upon nature of acid used. Now, let’s check our answer to see whether it’s reasonable. Finding ph of a buffer solution on addition of hydrogen iodide to it. P h = p k a + log.
8 × 1 0 − 5)
So the assumptions we make for a buffer solution are: Experts are tested by chegg as specialists in their subject area. Buffer capacity is the measure of a buffer's ability to resist ph change. (k b f o r n h 3 = 1.
That is, when ph = pk a.
When concentrations of both the salt and acid are halved, the ratio [acid][salt]. A buffer resists changes in ph due to the addition of an acid or base though consumption of the buffer. Answer:the effectiveness of a buffer depends upon two factors namely,(i) the amount of acid and its conjugate base relative to each other. Can anyone share the explanation, please?
Because different acids have different stren.
A higher buffer concentration has a greater buffer capacity. The buffer capacity depends essentially on 2 factors: The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes on the dilution or addition of a small amount of either acid or base. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit.
Degree of hydrolysis of ammonium acetate does not depend upon the concentration of ammonium acetate solution.
The ph of a buffer depends on two factors: That says the ph of a buffer solution does not change on the addition of a small amount of an acid or a base, it is true as buffer solutions are the. 3.range of ph scale is. 4.level of ph found in antacid solution
Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture.
The ph of a buffer depends on the ratio [base]/[acid] rather than on the particular concentration of a specific solution. The ph of the buffer solution depends upon the concentration of acid (h⁺) only conjugate base (oh⁻) only salt acid (h⁺) and conjugate base (oh⁻). This ability depends on the concentration of the buffer components, meaning the acid and its conjugate base. A neutralisation reaction in aqueous medium depends upon the number of equivalents of acid and base.
(ii) the absolute concentration of the acid and its.
The buffer capacity depends essentially on the ratio of the salt to the acid or base. [ a x −] [ h a] since concentration appears in both the numerator and denominator of the fraction [ a x −] [ h a] and p k a is constant (at a fixed temperature), it appears that dilution of the solution with pure h x 2 o would not change the p h. Ph of buffer solution depends upon concentration of salt both a and b answer is both a and b comments and discussions. Calculate the change in ph of one litre of buffer solution containing 0.10 mole each of n h 3 & n h 4 c l upon addition of, (i) 0.02 mole of dissolved gaseous h c l (ii) 0.02 mole of dissolved n a o h.
Biochemical engineering objective type questions and answers.
You don't need to login to post your comment.