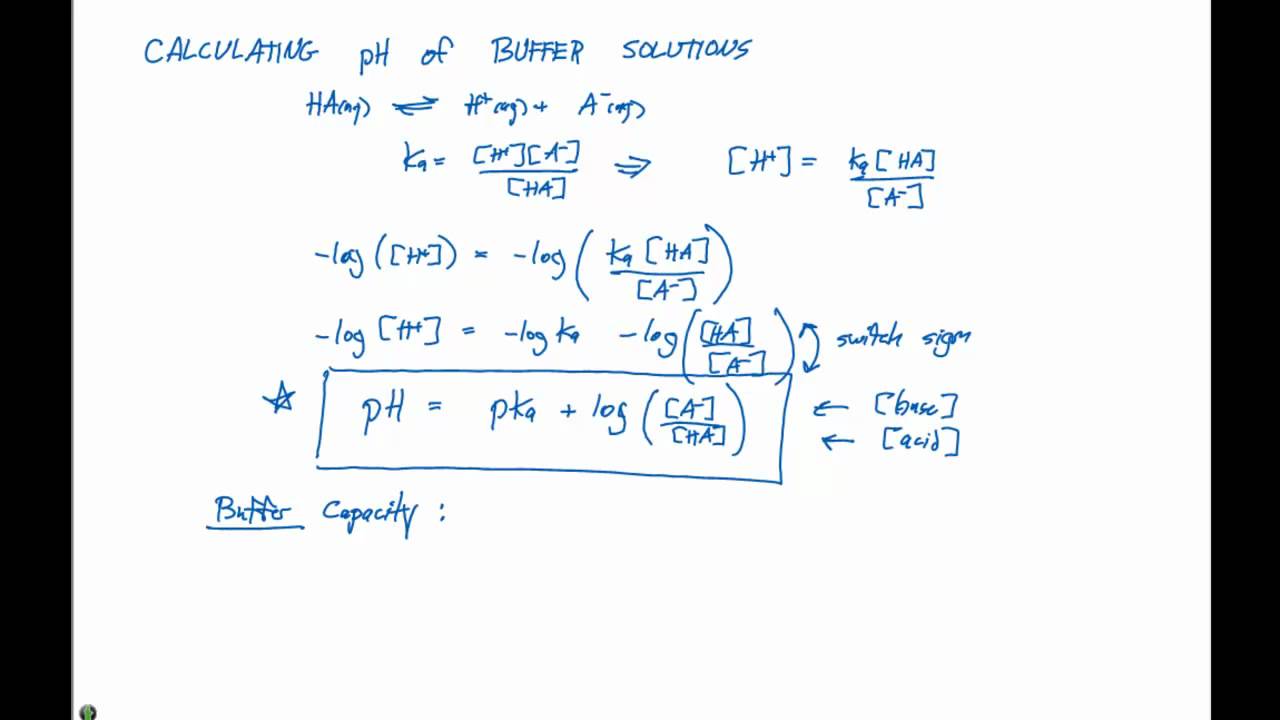
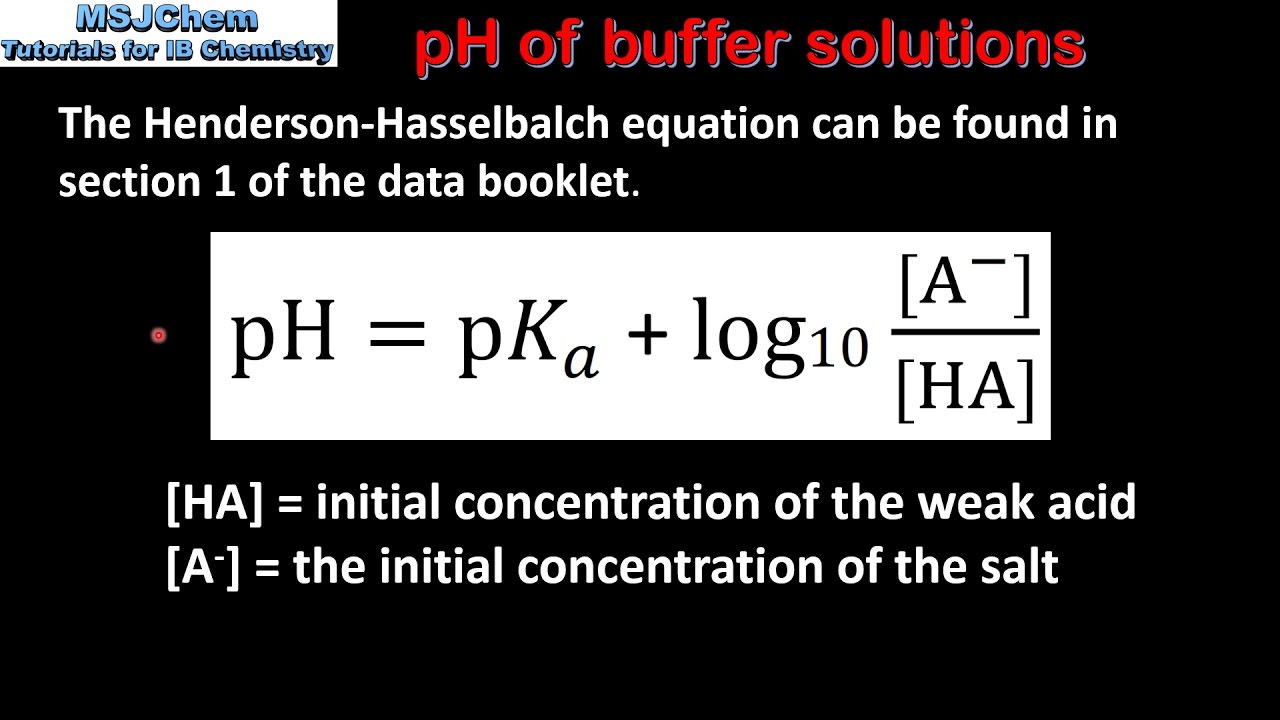
By knowing the k a of the acid, the amount of acid, and the amount of conjugate base, the ph of the buffer system can be calculated. Ad get quality measurements for your calibration procedures with our buffer solutions. Ka= acid dissociation constant ;
Buffer Solution Diagram Diagram Media
4.74 0.00 4.74 2.00 2.00 log log1.8 10 5 log = + = =− + = + − m m x.
Calculate the volume of 0.2m solution of acetic acid that needs to be added.
From the calculation above, the ph of buffer solution is 7.38. [ h x +] = k a × [ h a] [ a x −] =. Ad get quality measurements for your calibration procedures with our buffer solutions. Pk a (at 25°c) phosphate:
We can also use the alkaline buffer equation to calculate the ph but need to take note on the following points:
Solutions with a ph equal to 7 are. Ph of a buffer (henderson equation) calculator. Buffer calculations 1.0 what is the ph of 50.00 ml buffer solution which is 2.00m in hc2h3o2 and 2.00m in nac2h3o2? Taking negative logarithms of both sides, we obtain.
0.030 0.300 = 0.10 m o l d m − 3.
Principle, protocol & calculations a buffer solution (more precisely, ph buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid. Two types we must be able to handle: Calculate ph of a buffer solutionmicrobe notes by knowing the kaof the acid, the amount of acid, and the amountof conjugate base, the ph of the buffer system can be calculated. Now, let’s check our answer to see whether it’s reasonable.
[cb] = concentration of the conjugate base ;
Calculate the ph of an unbuffered 0.010m acetic acid solution. If the ph is higher than that number, the solution is basic, as known as alkaline. 0.020 0.300 = 0.067 m o l d m − 3. [ca] = concentration of the conjugate acid;
Decrease or increase depends on your frame of reference.