The 1s, 2p, 3d, and 4f aos kaino(ceno)symmetric (greek: Although the consequences of the very compact 2p shell (e.g. And the lanthanum atoms and their trivalent ions have the following general electron configuration.
Tikalon Blog by Dev Gualtieri
Gen atom, the order of increasing orbital energy is given by 1s < 2s = 2p < 3s = 3p = 3d, etc.
In particular, we emphasize the special role of the absence of a radial node whenever a shell with angular quantum number l is occupied for the first time (lack of “primogenic repulsion”), as with the 1s, 2p, 3d, and 4f shells.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for. 1s 22s 22p 63s 23p 64s 23d 10 4p 65s 24d 10 5p 6 (54 electrons in atom) rn: 2 electrons/orbital ↓ ↑ ↑ lili ↓ beb ↑↑ cn ↑ 3.) electrons occupy different orbitals of a given subshell before doubly occupying any one of them 4.) hund’s rule: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s.
In its ground state, an atom adopts a configuration with the greatest
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 5f 14 6d 10 7s 2 7p 6 2, 8, 18, 32, 32 18, 8 free gift for you: Germanium #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2. The element is in the 2nd column of the p block, group iva (column 13). Electrons will occupy different orbitals in a given subshell, before two electrons will occupy a single orbital.
For 2p, n = 2 and l = 1.
The radially nodeless 1s, 2p, 3d, 4f valence aos are particularly compact. For 4f, n = 4 and l = 3. I hope this was helpful. There is a simple way of remembering how electrons fill up orbitals, shown in the accompanying diagrams:
It is a common mistake to forget that the 4f sublevel is filled after the 6s sublevel and before the 5d sublevel.
Click to see full answer. Kainj&,kainos=new) and pyykkç[5] named them primogenic (latin:primus=first, genitus=born). All four of these orbitals have 0 nodes. The 3d orbitals have a double node at r2 ¼ 0 but no nodes at finite r.
All elements of the periodic table have exactly the same atomic orbitals.
The angular part produces its own node, which cuts through the same point. But in the ground state, every orbital above n = 1 is unoccupied. In these examples, each atom (other than helium) contains 8 valence electrons. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s.
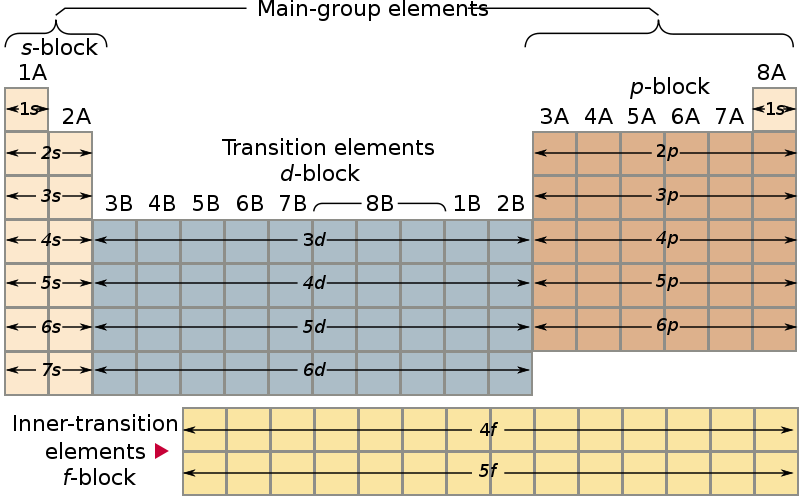
Notice that atomic numbers 57 through 70 on the periodic table below are in the 4f portion of the table.
6d 1s 2s 3s 4s 5s 6s 2p 3p 4p 5p. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 5d 4f 6p. Moving through the periodic table in this fashion produces the following order of sublevels up through 6s: Thus 2s lies below 2p, as already observed in helium.
The periodic table, and the unique chemical behavior of the first element in a column (group), were discovered simultaneously one and a half centuries ago.
Germainum is in the 4th row energy level of the periodic table. Periodic table group properties 1. Notice that atomic numbers 57 through 70 on the periodic table below are in the 4f portion of the table. Ogy, the 2p orbitals have only a ‘‘trivial’’ radial node at r ¼ 0.
Similarly, the 4f orbitals exhibit
1s 2, 2s 2, 2p 6, 3s 2 1s 2, 2s 2, 2p 6, 3s 2,3p 4 s, 16 electrons: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. Similarly, 3s, 3p and 3d increase energy in that order, and so on. Harris and jones[6] investigated the different geometric and electronic structures and the bondingingroup 14 dimers (c2 to pb2)and highlighted the nodelessnessofthe c2pshell.
Even a hydrogen atom has 1s, 2s, 2p, 3d, 4f, 9d, etc.
The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s. Periodic table_rev1[2] change bi k to c. For 1s, n = 1 and l = 0. Up to 10% cash back this paper describes the construction of the periodic tables for cations of all elements with charges + 1, + 2, + 3 and anions with charge − 1.
20 votes) the 4f block in the periodic table has been variously called rare earth, lanthanum's, and lanthanum.
The 1s orbital is the simplest orbital. For 3d, n = 3 and l = 2. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 (86 electrons in atom) note: [xe]4f˜ˆ5d˙6s˙ 3.20 hex 1.582 25.73 23.0.
1s < 2s = 2p 3d</strong> < 4s = 4p = 4d = 4f <.
119 rows 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2. 1.) order of orbital occupation: The radially nodeless 1s, 2p, 3d, 4f valence aos are particularly compact. The rows of the periodic table contain the following states.
The perodic table of elements is based on the fact that atoms with the same number of electrons outside a closed shell have similar properties.
It is a common mistake to forget that the 4f sublevel is filled after the 6s sublevel and before the 5d sublevel. Both hydrogen and helium atoms in the ground state have the simplest occupied orbital.