Most nonmetals do not react with air in room temperature. Poor conductors of electricity and heat; Nonmetals are elements that form negative ions by gaining electrons during chemical reactions.
NONMETALS METALS AND. Elements can be classified as
They gain or share electrons when they react with other elements and chemical compounds.
A few elements have properties that are either anomalous given their category, or otherwise extraordinary.
Most or some elements in each category share a range of other properties; Nonmetals tend to be softer, often colorful elements. Most nonmetals have the ability to gain electrons easily. In the solid state, nonmetals are brittle , meaning that they will shatter if struck with a hammer.
Chlorine is the second halogen, being a nonmetal in group 17 of the periodic table.
Usually, nonmetals react with other nonmetals in high temperature. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Like metals, nonmetals may occur in the solid, liquid, or gaseous state. Instead of dull in appearance.
Which physical properties are characteristic of nonmetal solids?
Under normal conditions, more than half of the nonmetals are gases, one is a liquid, and the rest include some of the softest and hardest of solids. Solid nonmetals are generally brittle, with little or no metallic luster. Chemically, nonmetals tend to have relatively high ionization energy, electron affinity, and electronegativity. Maybe solids, liquids or gases at room temperature;
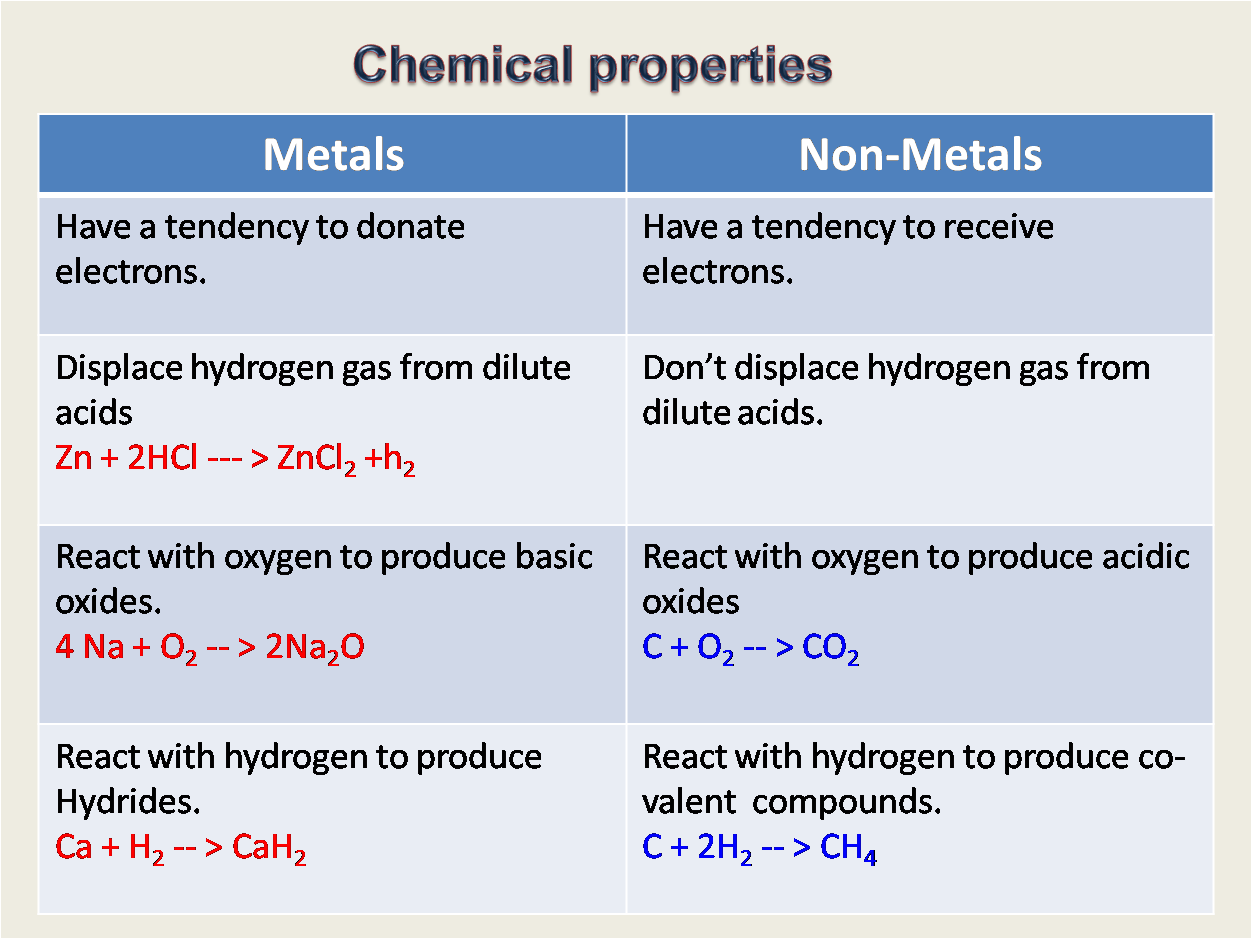
What are the chemical properties of non metals?
Since there is no rigorous definition of a nonmetal, some variation may be encountered among. They will not shine even if they are polished. However, unlike metals, nonmetals display a wide range of both mechanical and optical properties, ranging from brittle to plastic and from transparent to opaque. Start studying properties of nonmetals.
Its properties are thus similar to fluorine, bromine, and iodine, and are largely intermediate between those of the first two.
Nonmetals have high ionization energies and electronegativities. Melting points are generally much lower than those of metals. Of electricity a substance with a high density means it has a high mass for its size. These are electronegative elements with high ionization energies.
They tend to have lower melting points than metals.
Are poor conductors of heat and electricity; Nonmetals are poor conductors of heat and electricity. The solids are not lustrous. They cannot be drawn into wires.
They are generally poor conductors of heat and electricity.
Unlike metals, nonmetals aren't malleable and ductile. In brief one can say nonmetal is an element or a substance that is not a metal. Typical nonmetals have a dull, coloured or colourless appearance; A nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity.
Nonmetals display a wide range of chemical properties and reactivities.
It means they cannot hold heavyweights. Their properties and behavior are quite different from those of metals on the left side. The nonmetals are elements located in the upper right portion of the periodic table. High ionization energy and do not give up electrons easily.
Define the properties of nonmetals.
Nonmetals possess the following properties in general: What are 3 physical properties of. Thus, they are electronegative elements with high ionization energies. Nonmetals react more with metals than with nonmetals.
The periodic table consists of elements that are metals, those that are nonmetals, and elements with properties intermediate between the two groups (metalloids).
And is also a good conductor. Describe structure and properties of nonmetals.