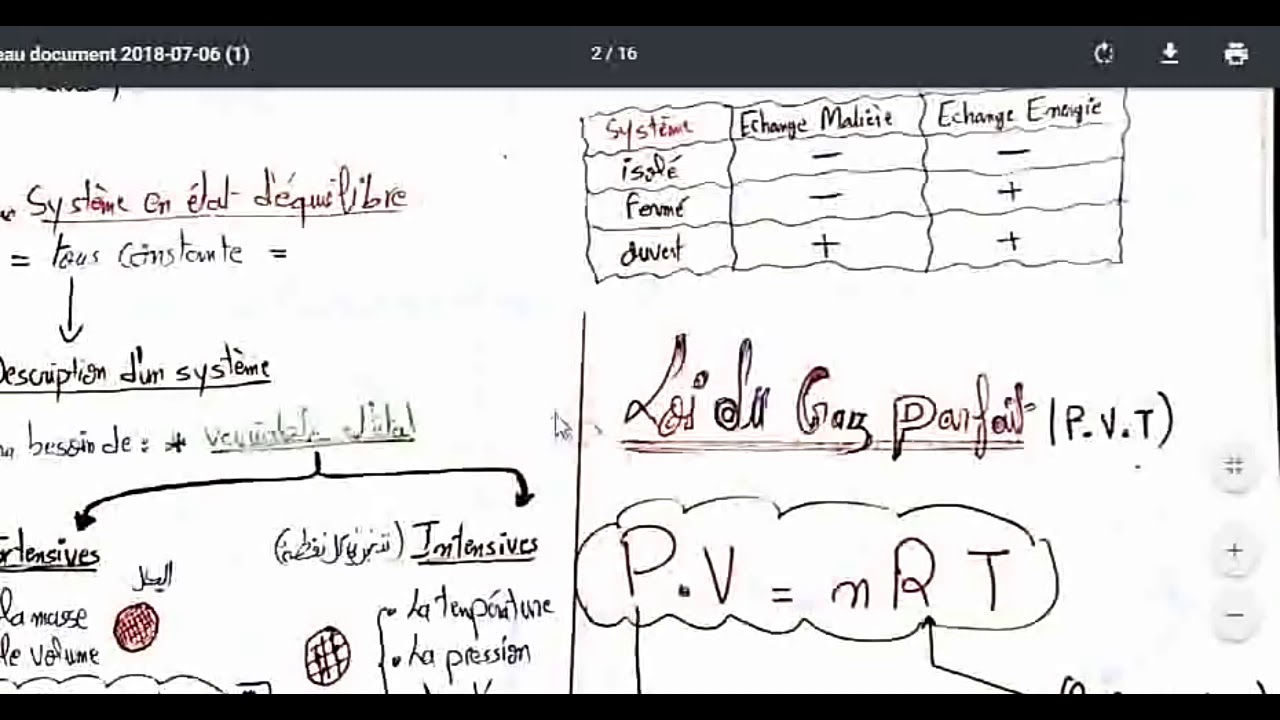
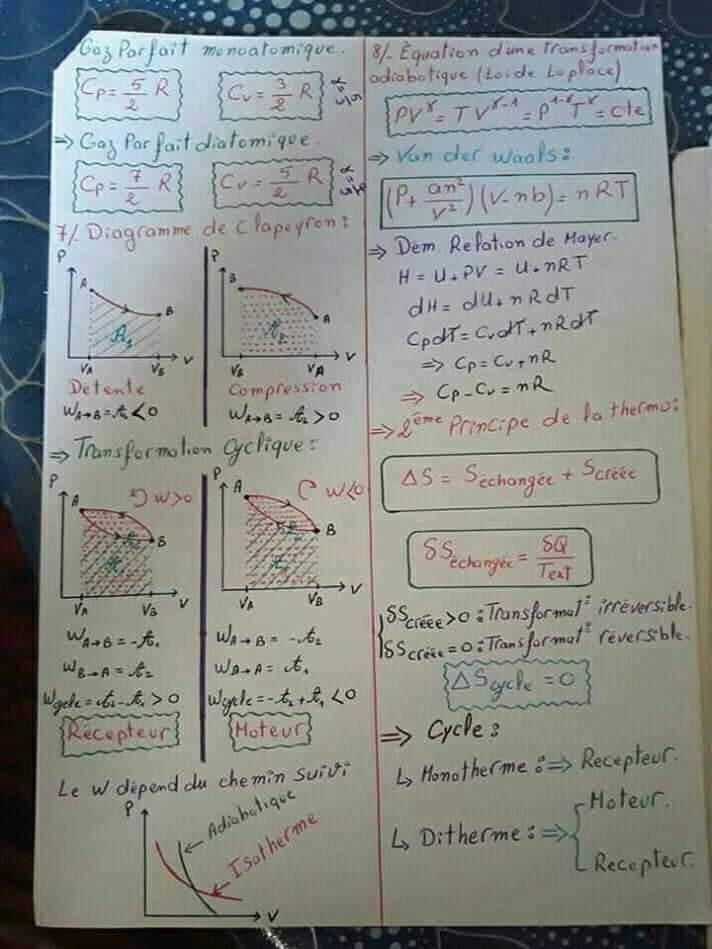
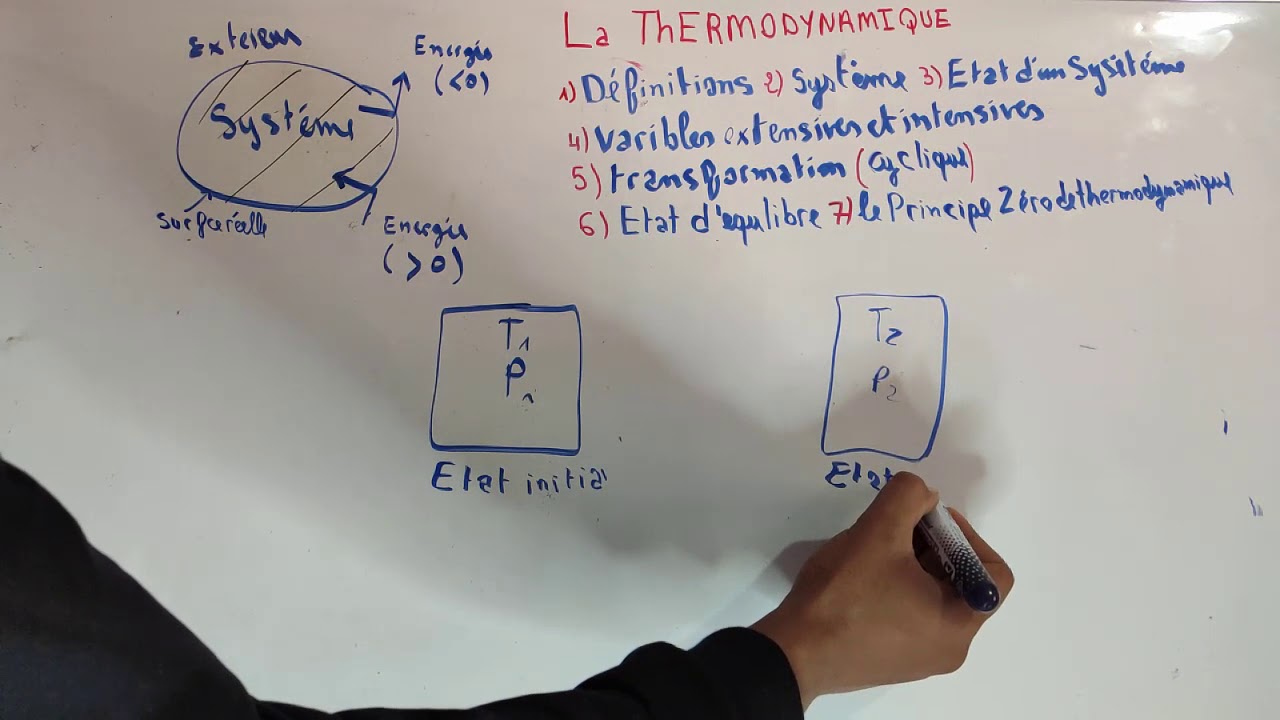
Fiche 6).à l’aide d’un thermomètre à gaz (gaz réel sous faible pression), et en expérimentant à Cours thermodynamique smpc s1 on peut définir la thermodynamique de deux façons simples : Cours thermodynamique smia s1 pdf amil tayyar operasyon ergenekon pdf la tetralogie de fallot pdf fantasia on themes from la traviata pdf bret easton ellis the informers pdf.
thermodynamique s1 YouTube
Classreport.org provided free website for the class of 1979 from martin luther king high school for the members and guests of this class to stay informed of reunion events and updates from fellow members.
(opens a modal) thermodynamics part 2:
(this is an experimental fact!) (vw, s & b: W e can change the state of the system in two different ways. Jj508 engineering laboratory 3 thermodynamics 2 experiment 1 heat transfer 1 2. Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
The behaviour of these quantities is governed by the 4.
Jpg il peut absolument effectuer la majorité des tâches de ce dernier de manière efficace et optimale. (opens a modal) thermodynamics part 5: If you're seeing this message, it means we're having trouble loading external resources on our website. Explanation are given for understanding.
It was born in the 19th century as scientists were first discovering how to build and operate steam engines.
La seconde est venue ensuite, grâce aux travaux pionniers de ludwig boltzmann. (opens a modal) thermodynamics part 3: Thermodynamics 157 internal energy of the system in state a be called u a. (opens a modal) thermodynamics part 4:
Let's assume a fluid of thermal convection h circulate from the left to the right across a shell.
We do some mechanical work, say 1 kj, by rotating a set of small paddles and Thermodynamics mcq question with answer thermodynamics mcq with detailed explanation for interview, entrance and competitive exams. Kelvin scale and ideal gas law example. Its rate d is the quantity of fluid, per unit time, crossing the surface s 1 = π r 1 2.at the entrance, the fluid has a temperature t hi and at the exit, it becomes less hot and has a temperature t co (t hi > t co).if the specific heat at constant volume of the fluid is c v, and the.
Fundamentals of thermodynamics, sonntag, borgnakke, and van wylen, 2003, 6th edition, john wiley and sons.
Small scale gas interactions are described. La première définition est aussi la première dans l'histoire. Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation.the behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms. Thermodynamics is a branch of physics which deals with heat and temperature and their relation to energy and work.
At the end of the lab session students should be able:
Molar ideal gas law problem. To understand the use of the fourier rate equation in determining rate of the heat flow through solid material for one dimensional, steady flow heat. La science de la chaleur et des machines thermiques ou la science des grands systèmes en équilibre. Thermodynamics is a branch of physics which deals with the energy and work of a system.
Pour communiquer avec le professeur propriétaire de la leçon :0627846439 pour me contacter sur instagrame :
Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. Moles and the ideal gas law.