Gases (oxygen) and solids (carbon). Metals are on the left of the line, in blue. Strong, malleable and ductile, react with oxygen to form basic oxides, sonorous, high melting and boiling points, good conductors of electricity, good conductors of heat, mainly solids at room temp.
ENGLISH ON THE NET PROPERTIES OF METALS AND NON METALS
For example, sulphur and phosphorus occur in solid state while bromine occurs in liquid state.
* properties of metals physical properties:
What are 3 physical properties of nonmetals? Thus, they are electronegative elements with high ionization energies. They tend to have lower melting points than metals. Properties of nonmetals they also have a low melting point.
Metals (like copper and aluminium) are good conductors of heat and electricity, while nonmetals.
Properties of nonmetals nonmetals have low density. Brittle, react with oxygen to. Hydrogen, helium, oxygen, nitrogen, fluorine, neon or radon and many others. 10 rows most metals are solids at room temperature with a characteristic silvery luster (with the.
Main group al, ga, in, sn, tl, pb, bi, po.
Au and cu good conductors metallic luster (shine) malleable ductile chemical properties: , shiny when polished, high density, eg. Alkali elements li, na, k, rb, cs, fr Lose electrons (makes positive ion) corrode easily * properties of.
Most metals are hard, except sodium.
On reacting with the oxygen they give rise to acidic or neutral oxides. 11 rows a nonmetal is simply an element that does not display the properties of a metal.it is not. On reacting with water they give rise to hydroxides and hydrogen gas. Nonmetals are on the right of the line, in orange.
Metals refers to the natural elements that are hard, shiny, opaque and dense.
Properties of metals and nonmetals worksheet. All the metals are good conductors of heat and electricity. Some of the worksheets below are properties of metals and nonmetals worksheet : Elements like sulphur, carbon, oxygen etc.
Nonmetals are elements that form negative ions by gaining electrons during chemical reactions.
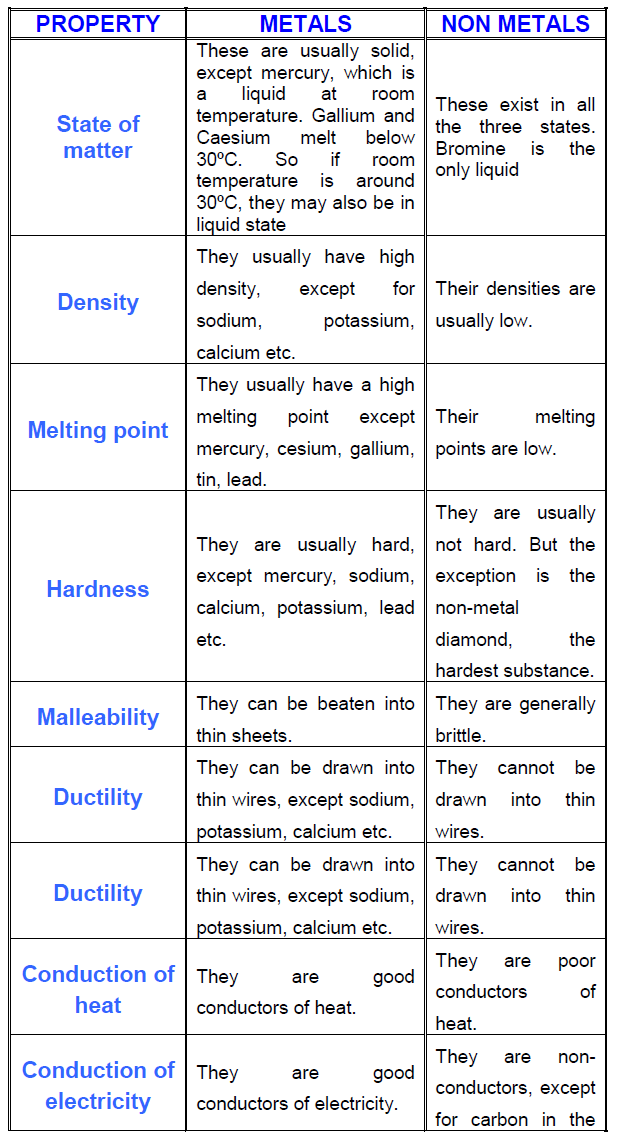
Vocabulary, physical properties of metals, questions like identify each element as a metal. This is why they are poor conductors of heat and electricity. Except for sodium, calcium and potassium metals do not combine with hydrogen. The characteristic properties of metals and nonmetals are quite distinct, as shown in the table below.
A non metal is also a good insulator for heat and cold.