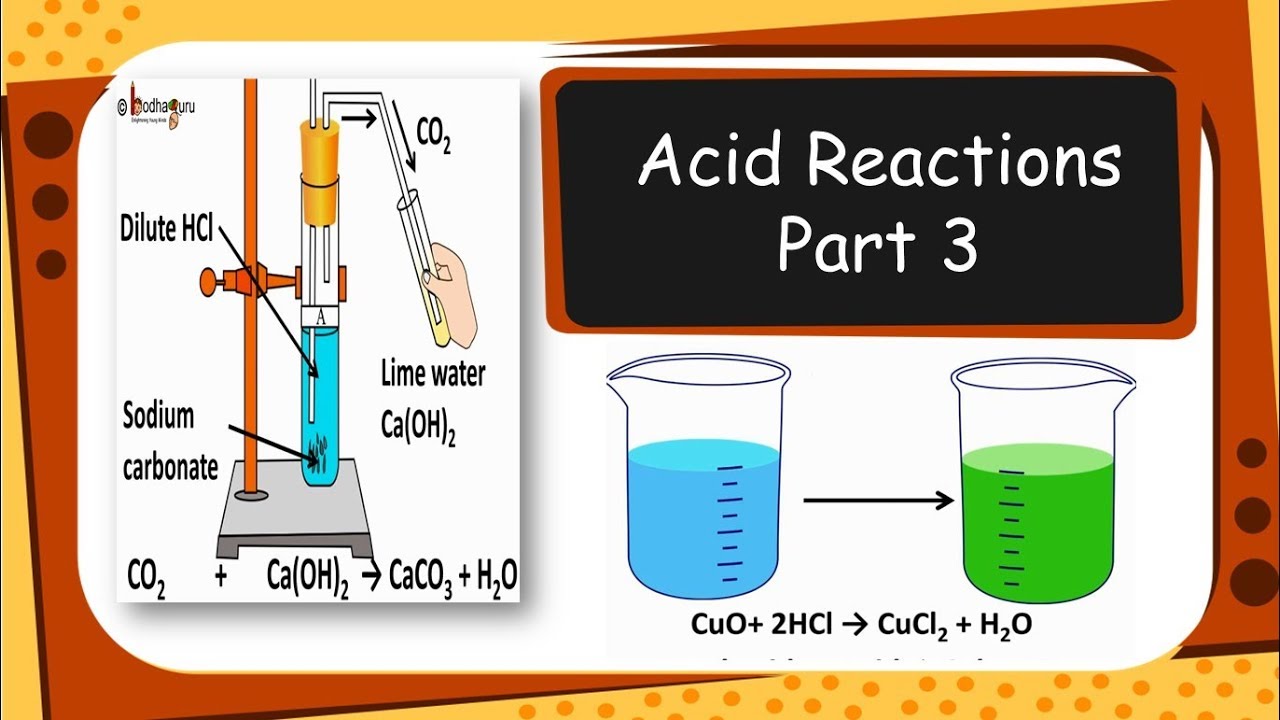
Na2o + 2 hcl → 2 nacl + h2o. The blue colour of the solution indicates the formation of copper (ii) sulphate. Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature.
Difference Between Acidic and Basic Oxides Definition
Metal oxides are basic in nature.
\(2\text{hcl (aq)} + \text{na}_{2}\text{o (aq)} \rightarrow \text{h}_{2}\text{o (l)} + 2\text{nacl}\)
Raju while cleaning the floor with hydrochloric acid observing the bottle. Metal oxide and acid equations. Acid + metal oxide → salt + water. These salts have a basic behavior, hence their name.
Thus, when an acid reacts with a metal oxide both neutralize each other.
Most metal oxides react with water to form metal hydroxides. Acid + metal hydroxide → salt + water. Write balanced equations for the following acidic reactions. He got a doubt the compound is formed.
When an acid reacts with an alkali salt (a metal hydroxide), the product is a metal salt and water.
H 2 so 4 (aq) + cuo(s) → cuso 4 (aq) + h 2 o(l) reactions with metal hydroxides The reaction between copper oxide and nitric acid forms copper nitrate and water. Acid + metal oxide/metal hydroxide → salt + water. We can write a general equation for the reaction of a metal oxide with an acid:
In this reaction, salt and water are formed.
Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature. When an acid (such as hydrochloric acid) reacts with calcium oxide,. Magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. Naoh(aq) + hno 3 (aq) nano 3 (aq) + h 2 o(l) a comment about these reactions and the reactivity series
Acid + metal oxide salt + water.
The black powder copper oxide is an example of a metal oxide. Acid reacting with metallic oxides and metal hydroxide. Reaction of acid with metal oxides: A cut and stick activity for an sen class / lower ability to learn how to write chemical equations for the reaction between metal oxides (bases) with hydrochloric, sulfuric and nitric acids.
The general reaction can be written as follows:
Acids react with metal oxides to produce a salt and water. With metal oxides and hydroxides, nitric acid behaves in exactly the same way as the other acids, this time producing nitrates. What is the reaction between acetic acid and metal oxide powder? When a metal carbonate reacts with an acid, a salt, water and carbon dioxide form as products.
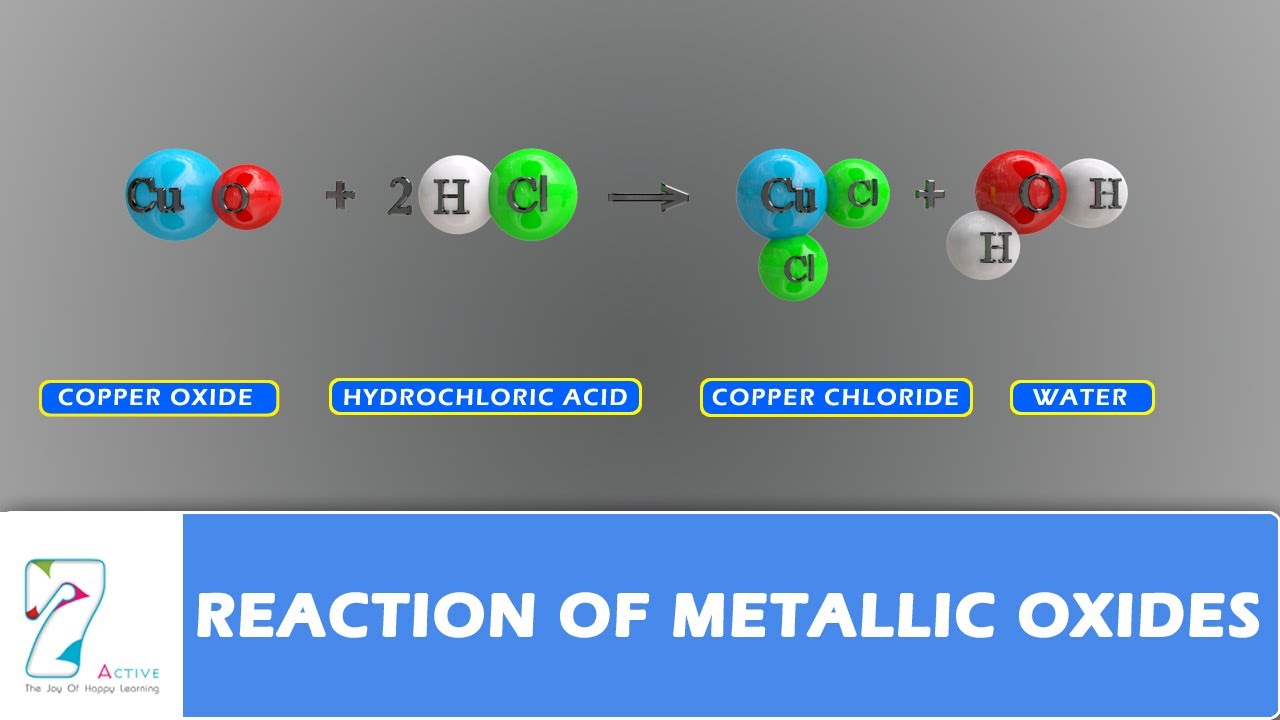
Let us consider the reaction of copper oxide with dilute hydrochloric acid.
The general word equations for the reactions of this chapter are the following: Na2o + 2 hcl → 2 nacl + h2o. Naoh + hcl → nacl + h2o. Most metal oxides react with water to form metal hydroxides.
Acids react with metal oxides to form salt and water.
When an acid reacts with a metal, the products are a salt and. Cuo(s) + 2hno 3 (aq) cu(no 3) 2 (aq) + h 2 o(l). So, for example, with copper(ii) oxide: Acid + metal oxide → salt + water.
9 rows acids react with most metals.
The metal oxides , also known as basic oxides, are compounds produced by the reaction of a metal with oxygen. M 2 + 2hcl → 2mcl + h 2 or. When dilute hydrochloric acid reacts with copper (ii) oxide (cuo) , it forms a salt called copper (ii) chloride (cucl 2 ) and water (h 2 0). Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water.
Some examples of reactions between acids and metal oxides are copper oxide with hydrochloric acid and the combination of copper oxide with nitric acid.
In this reaction, salt and water are formed. And with sodium hydroxide solution: Which gas is usually liberated when an acid reacts with a metal? Metal oxides contain atoms of a metal combined with atoms of oxygen.
Thus, when an acid reacts with a metal oxide both neutralize each other.
Acid + metal oxide salt + water. In the first reaction, the copper oxide reacts with hydrochloric acid to produce copper chloride and water. 2yh+(aq) + mxoy(aq) → yh2o (l) + xmn+(aq) 2 y h + (aq) + m x o y (aq) → y h 2 o (l) + x m n + (aq) where n n is the group number of the metal. Mg(s) + h2so4(aq) mgso4(aq) + h2(g) the reaction with dilute hydrochloric acid looks exactly the same, but this time magnesium chloride is produced.
Acid + metal oxide ⇨ salt + water.
For example, when a small amount of copper oxide is added to sulphuric acid, colour of the solution becomes blue and the copper oxide dissolves. Magnesium (mg) and hydrochloric acid (hcl) 2. Acid and metal oxide (esbr2) when an acid reacts with a metal oxide a salt and water are also formed. Metallic oxides react with acids to give salts and water.
Na2o + h2o → 2naoh.
Na2o + h2o → 2naoh. Acids react with a metal to produce a salt. The salt produced from these reactions is dependent on the acid that is used. Metal oxides are basic in nature.
Metal hydroxides then reaction with acids to form salts and water.
Acid + metal oxide salt + water. Naoh + hcl → nacl + h2o. Metal oxides are basic in nature. This can be shown in an equation:
Acid + metal carbonate → salt + water + carbon dioxide.
Metal oxides react with acids to produce a salt and water. In summation, a metal oxide will react with an acid to form water and a salt. Reactivity of acids with metal oxides. Acids react with metal oxides to produce a salt and water.
Reaction of acid with metal oxides:
Acid + metal oxide → salt + water. This proves that metallic oxides are basic oxides. Magnesium oxide, a metal oxide. Zinc (zn) and hydrochloric acid (hcl.
Help him by explaining how hcl molecule formed?