An alkaline solution can be formed when a metal oxide is dissolved in water. A metal salt is obtained as a product and hydrogenalso. When reacting with water, these compounds form oxacid acids , but if they are in the presence of hydroxides , what is.
Acidity of different metal oxide pillared montmorillonites
In the first reaction, the copper oxide reacts with hydrochloric acid to produce copper chloride and water.
Cuo(s) + h 2 so 4 (aq) cuso 4 (aq) + h 2 o(l) oxide ions from the copper(ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer.
The general equation for reacting a metal oxide with an acid is: Metal oxides are one of the most important and widely characterized solid catalysts. When an acid reacts with a metal, the products are a salt and hydrogen. Acids react with metal oxides to produce a salt and water.
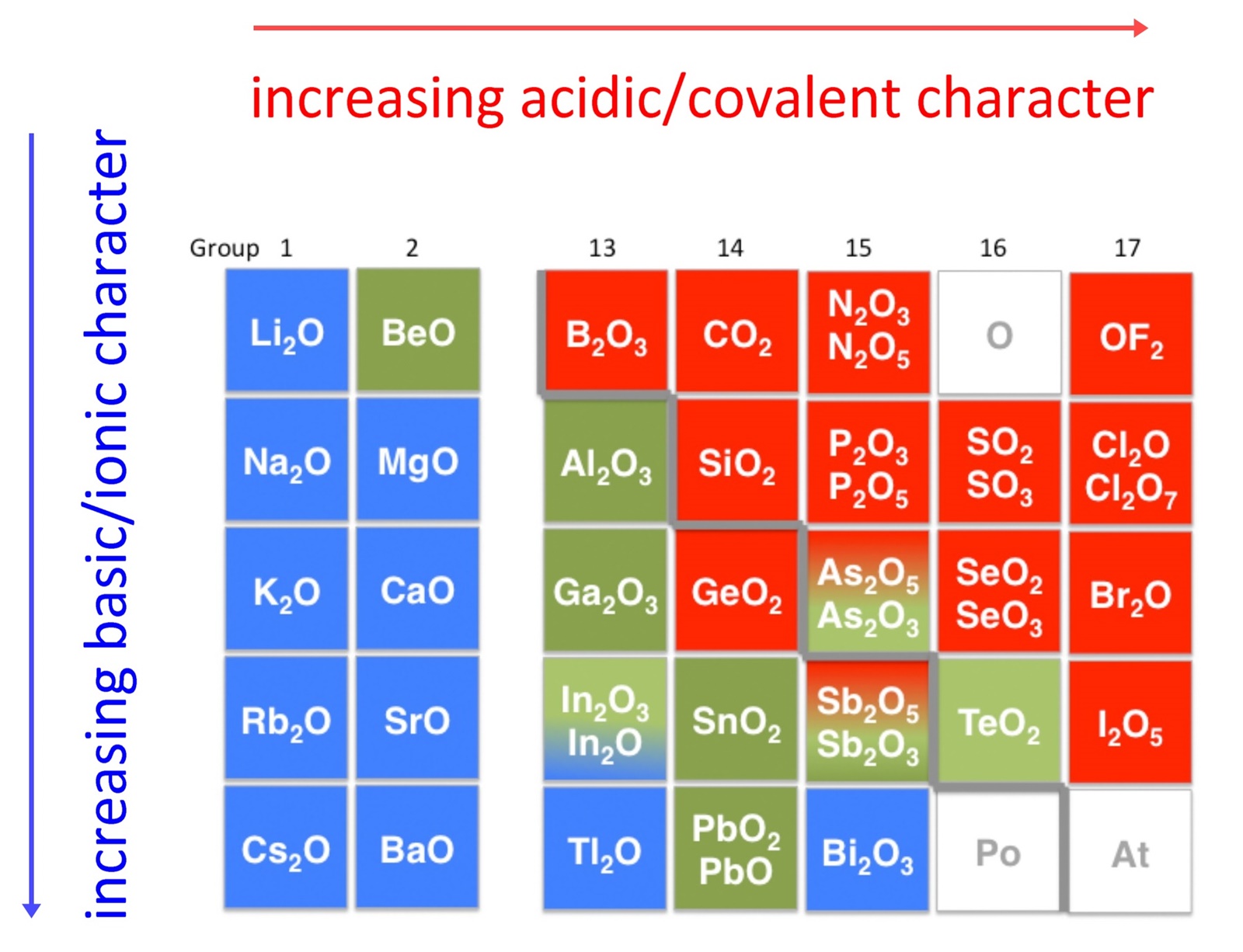
Oxides like this are described as basic oxides.
2yh+(aq) + mxoy(aq) → yh2o (l) + xmn+(aq) 2 y h + (aq) + m x o y (aq) → y h 2 o (l) + x m n + (aq) where n n is the group number of the metal. Metal + acid → salt + hydrogen The salt produced from these reactions is dependent on the acid that is used. Some examples of reactions between acids and metal oxides are copper oxide with hydrochloric acid and the combination of copper oxide with nitric acid.
Metal oxide acid salt total:
The overall equation for the reaction between copper(ii) oxide and dilute sulfuric acid is. This can be shown in an equation: How do metal oxide react with acid? Acid + metal carbonate → salt + carbon dioxide + water.
The type of salt that forms will depend on the specific acid and metal oxide which were used in the reaction.
Acid anhydrides usually have a low melting and boiling point except for compounds like b 2 o 3 and sio 2 which have high melting points and form giant molecules. Metal oxides form chloride salts when reacting with hydrochloric acid, nitrate salts when reacting with nitric acid and sulfate salts when reacting with sulfuric acid. 2hcl + mgo → mgcl 2 + h 2 o. Metal oxides react with acids to produce a salt and water.
This is the general word equation for the reaction:
Let’s learn about the reaction of metallic oxide with an acid and of non metallic oxide with base Acids react with most metals. Chemsrc provides metal oxide acid salt's classification, including all chemical products obtained by chemical processes (chemical methods to change the composition or structure of substances, or to synthesize new substances, all of which belong to chemical production techniques). Rodrigez@bnl.gov abstract this chapter covers the fundamental science,.
Hcl + naoh → nacl + h 2 o.
Kennedy, 16129 genova, italy abstract the ir spectroscopic methods for the characterization of the surface acidity of metal oxide catalysts are brie¯y reviewed. Acid + metal oxide salt + water. Acid + metal oxide salt + water. Catalysis today 41 (1998) 191±206 spectroscopic characterization of the acid properties of metal oxide catalysts guido busca* istituto di chimica, facoltaá di ingegneria, universitaá ± p.le j.f.
The reaction between copper oxide and nitric acid forms copper nitrate and water.
The x x and y y represent the ratio in which. Since the base in our reaction is a metal oxide we can write: Sulfur dioxide and nitrogen dioxide however, will dissolve to form acidic solutions. This is the general word equation for the reaction between an acid and a metal oxide.
Zinc nitrate is produced by carrying out a reaction between the following metal oxides and acid.
These compounds can also be called as acid anhydrides. Certain groups of metals, particularly transition metals, have attracted much attention because of their outer electron configuration. Acid + metal oxide → salt + water. Similarly, when metal hydrogen carbonate like nahco 3 reacts with dilute hydrochloric acid, salt and water are formed with the evolution of carbon dioxide.
So,this reaction is similar to a neutralization reaction.
Soluble metal oxides produce alkalis when dissolved in water. Acid + metal oxide → salt + water. Since the difference in electronegativity between these elements is low, the bonds that are formed between them are covalent. The general equation for this is, acid + metal oxide.
Metal oxides are bases, because they neutralise acids.
We can write a general equation for the reaction of a metal oxide with an acid: There are various ways in. A salt and water are produced when acids react with metal oxides. 2hcl + caco 3 → cacl 2 + co 2 + h 2 o.
When an acid reacts with a metal oxide a salt and water are also formed.
Metal oxides are basic in nature like the metal hydroxides. Generally metal oxides are soluble in acids but this is notmandatory. Acid + metal → salt + hydrogen gas The reaction of an acid with a metal oxide gives rise to a corresponding salt and water.
/GettyImages-1167557769-49aed7c48b064ff4ba8419cf8ef7aa6e.jpg)