What is the reaction between acetic acid and. How do metal oxides react with acids and water? Metal oxides react with acids to produce a salt and water.
PPT Preparation of Salts PowerPoint Presentation ID232318
Na2o + h2o → 2naoh.
∙ the salt that is produced depends upon acid and metal reacting.
Sulfuric acid + copper oxide → copper sulfate + water. The reaction between copper oxide and nitric acid forms copper nitrate and water. Calcium is metal and calcium oxide is a metallic oxide which is basic in nature. Na2o + 2 hcl → 2 nacl + h2o.
Metal oxides are basic in nature.
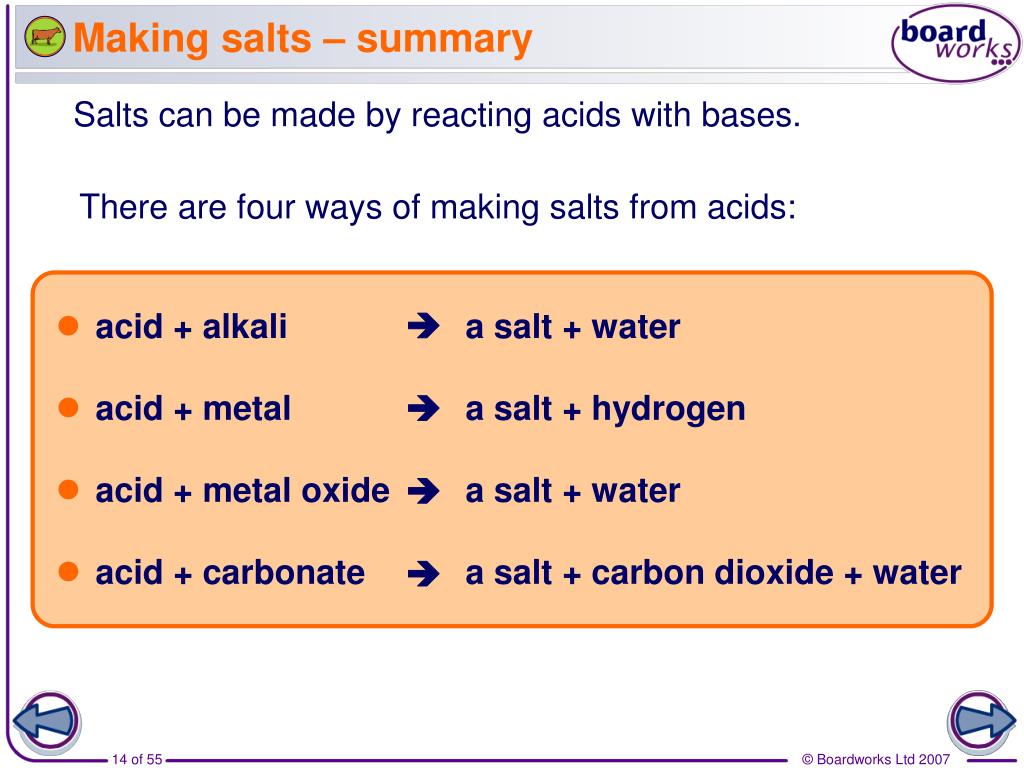
Acid + metal oxide ⇨ salt + water. When reacting with water, these compounds form oxacid acids , but if they are in the presence of hydroxides , what is. Since the difference in electronegativity between these elements is low, the bonds that are formed between them are covalent. In summation, a metal oxide will react with an acid to form water and a salt.
When reacting with water, these compounds form oxacid acids, but if they are in the presence of hydroxides, what is formed is a salt and water.
2hcl (aq) + na2o (aq) → h2o (l)+ 2nacl 2 hcl (aq) + na 2 o (aq) → h 2 o (l) + 2 nacl. H 2 so 4 (aq) + cuo(s) → cuso 4 (aq) + h 2 o(l) reactions with metal hydroxides Like any base, these bases can be neutralized by an acid to form a salt and water. Metal oxides are basic in nature.
Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature.
Na2o + h2o → 2naoh. When an acid reacts with a metal oxide a salt and water are also formed. Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature. Metal oxides) to produce salts and water base + acid salt + water example:
2hbr (aq) + mgo → h2o (l) + mgbr2(aq) 2 hbr (aq) + mgo → h 2 o (l) + mgbr 2 (aq)
In these types of reactions respective salt and water are formed. Some examples of reactions between acids and metal oxides are copper oxide with hydrochloric acid and the combination of copper oxide with nitric acid. In the first reaction, the copper oxide reacts with hydrochloric acid to produce copper chloride and water. Most metal oxides react with water to form metal hydroxides.
Metal hydroxides then reaction with acids to form salts and water.
The correct answer is option 1 i.e metal oxide + acid ⇨ salt + water. Reaction of acid with metal oxides: The salt produced from these reactions is dependent on the acid that is used. Metal + acid respective salt + water example :
In this reaction, salt and water are formed.
To learn more about identification, examples, faqs with videos of amphoteric oxide,. Acids react with metal oxides to produce a salt and water. Acid + metal oxide → salt + water. When an acid reacts with a metal oxide they both neutralize each other.
This can be shown in an equation:
The new compounds formed are salt and water. The general equation for reacting a metal oxide with an acid is: Copper oxide + sulfuric acid copper sulfate + water cu + h2so4 cuso4 + h2o zinc hydroxide + nitric acid zinc nitrate + water zn(oh )2 + 2hno3 zn(n o3)2 + 2h2o neutralisation reactions the particular salt produced in any reaction between an acid and a base or alkali depends on: The black powder copper oxide is an example of a metal oxide.
Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water.
Thus, when an acid reacts with a metal oxide both neutralize each other. ∙ acids react with metals to form salt and hydrogen gas. Acid + metal oxide salt + water. Acid + metal oxide salt + water.
Most metal oxides react with water to form metal hydroxides.
Metal oxides contain atoms of a metal combined with atoms of oxygen. In practice these substances are often made by reacting metal compounds such as oxides with the acid. For example, oxygen reacts with solid carbon to form carbon monoxide or carbon dioxide, respectively, as shown below (reaction not balanced): Each acid gives its own set.
Arsenic oxide, carbon dioxide, tellurium oxide.
Amphoteric oxides are classified as metal oxides that react with both acids as well as bases to create salts and water. Metal oxides are basic in nature. Acids give water along with respective salt when they react with a metal oxide. When an acid (such as hydrochloric acid) reacts with calcium oxide,.
Acid + metal oxide ⇨ salt + water.
In theory, a metal salt is a compound formed when the hydrogen of an acid is replaced by a metal. So if an acid reacts with a metal oxide neutralization reaction takes place. For example, copper oxide (cuo), sodium oxide (na 2o), magnesium oxide (mgo). There are various ways in.
Naoh + hcl → nacl + h2o.
H 2 so 4 (aq) + cuo(s) → cuso 4 (aq) + h 2 o(l) alkalis are soluble bases. Na2o + 2 hcl → 2 nacl + h2o. Acid + metal oxide ⇨ salt + water. Several of these compounds are gaseous substances.
Metal hydroxides then reaction with acids to form salts and water.