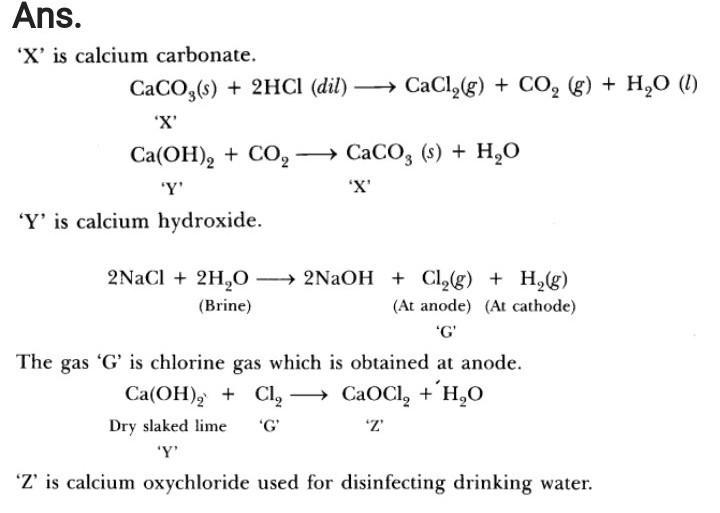
What happens when a metal oxide reacts with a dilute acid? The reaction between a metal oxide and an acid produces a salt and water. This proves that metallic oxides are basic oxides.
Acid Reaction With Metal Example
Acid + metal oxide salt + water.
Acid + metal oxide salt + water.
Which you get depends on the concentration. C a ( s) + 2 h 2 o ( l) → c a ( o h) 2 ( a q) + h 2 ( g) calcium floats over water during the reaction with water. All metal oxides react with water to give metal hydroxides and not salt and acid. So the nitrate ions oxidise metal atoms to give metal ions, but don't give hydrogen as well.
The metal replaces the hydrogen.
Metal oxides form chloride salts when reacting with hydrochloric acid, nitrate salts when reacting with nitric acid and sulfate salts when reacting with sulfuric acid. Metal oxides are basic nature. In this reaction, salt and water are formed. Metal oxides are basic in nature.
When a substance reacts chemically, both as a base or acid it termed as an amphoteric solution.
Thus, when an acid reacts with a metal oxide both neutralize each other. When an acid reacts with a metal, the products are a salt and hydrogen. As a result, salt is formed. Metal oxides react with acids to produce a salt and water.
Following equation shows this reaction.
Metal oxides are crystalline solids that contain a metal cation and an oxide anion. Reaction of acid with metal oxides: Acid + metal oxide ⇨ salt + water. If it is ferrous oxide (feo), the products are ferrous sulphate and water.
Reactions between metal oxides and dilute acids usually need to be heated gently.
The kind of salt produced is dependent on the specific acid used. A basic oxide is an oxide which when combined with water gives off a base. The salt produced from these reactions is dependent on the acid that is used. They typically react with water to form bases or with acids to form salts.
The x x and y y represent the ratio in which the metal combines with the oxide and depends on the valency of the metal.
The two products are salt and water. The reaction of calcium with water is less violent. Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature. Acidic nature of aluminium oxide:
This can be shown in an equation:
Oxide ions from the copper(ii) oxide and hydrogen ions from the acid have combined to make water and aren't there any longer. This is the general word equation for the reaction: The heat evolved is not sufficient for the hydrogen to catch fire. So, the correct option is (b) all metal oxides react with water to give salt and acid.
You will notice that the type of salt produced depends on the acid used.
Metallic oxides are basic in nature because they react with dilute acids to form salt and water. Aluminium oxide gives sodium aluminate along with water, when it reacts with sodium hydroxide. When an acid is treated with a metal oxide, a salt and water are obtained. This is the currently selected item.
Acidic, basic, and amphoteric oxides.
Basic nature of aluminium oxide: Metal oxide are acidic in nature. H 2 so 4 (aq) + cuo(s) → cuso 4 (aq) + h 2 o(l) reactions with metal hydroxides The bubbles of hydrogen gas formed stick to the surface of the water calcium.
The resulting mixture is warm.
Some metals react with acids to give salt and hydrogen. Hydrogen gas and sodium chloride are formed when hydrochloric acid reacts with sodium metal. When an acid (such as hydrochloric acid) reacts with calcium oxide,. Some examples of reactions between acids and metal oxides are copper oxide with hydrochloric acid and the combination of copper oxide with nitric acid.
When metal oxide reacts with dilute acid does it form?
In this reaction, salt and water are formed. Acid + metal oxide → salt + water. Acids react with metal oxides to produce a salt and water. What happens when metal oxide reacts with acid give example?
For example, when a small amount of copper oxide is added to sulphuric acid, colour of the.
Al 2 o 3 + 2naoh ⇨ 2naalo 2 + h 2 o. 2yh+(aq) + mxoy(aq) → yh2o (l) + xmn+(aq) 2 y h + (aq) + m x o y (aq) → y h 2 o (l) + x m n + (aq) where n n is the group number of the metal. Feo + h2so4 = feso4 + h2o. That leaves the copper(ii) ions and the sulfate ions to make the salt copper(ii) sulfate.
Reactivity of acids with metal oxides.
Following equation shows this reaction. M etals oxides will react with dilute acids to give a salt and water. The reaction between a metal oxide and a mineral acid is a simple neutralization reaction whose products are salt and water. Group 1 & 2 oxides are highly alkaline in nature that is why group 1 called alkaline metals and.
Neutral oxide is one which neither has an acidic characteristic or a basic one.
(b) all metal oxides react with water to give salt and acid metal oxides. Mo + h2o → m (oh)2 (where m = group 2 metal) thus, these compounds are often called basic oxides. Calcium is a metal, thus calcium oxide is a metallic oxide which is basic in nature. As for example, co2 is acidic in nature and na2o is basic in nature.
We can write a general equation for the reaction of a metal oxide with an acid:
Hydrogen gas and zinc chloride are formed when hydrochloric acid reacts with zinc metal. Aluminium oxide gives aluminium chloride along with water, when it reacts with hydrochloric acid. Metallic oxides react with acids to give salts and water. Identify the nature of metal oxide and non metal oxide.