Thus, they are electronegative elements with high ionization energies. Metals are malleable and ductile. Nonmetals can form charged ions by.
Metals and NonMetals Introduction Edusaint Courses
Metals refers to the natural elements that are hard, shiny, opaque and dense.
Vocabulary, physical properties of metals, questions like identify each element as a metal.
Most metals are solids at room temperature with a characteristic silvery luster (with the exception of mercury, which is a liquid). Properties of metals and nonmetals worksheet. The density of metals is usually high. Nonmetals are (usually) poor conductors of heat and electricity and are not malleable or ductile;
* properties of metals physical properties:
Typically, the outer shells of nonmetals comprise 4 to 8 electrons. , shiny when polished, high density, eg. Some of the worksheets below are properties of metals and nonmetals worksheet : Some metals react with air and corrode.
They tend to have lower melting points than metals.
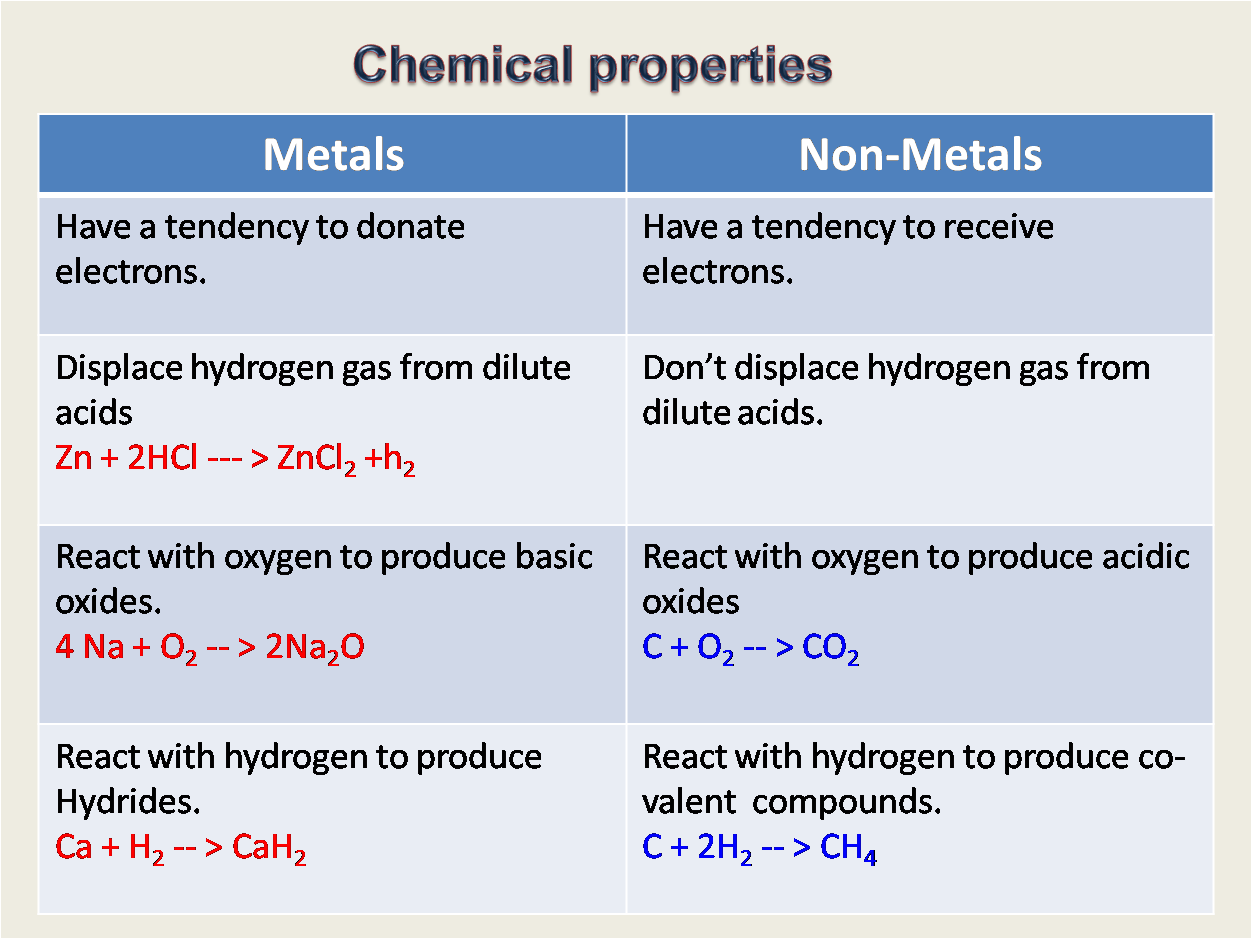
Brittle, react with oxygen to form acidic oxides, dull sound when hit with. Metals are sometimes represented by a cloud of delocalized electrons as a lattice of positive ions. Strong, malleable and ductile, react with oxygen to form basic oxides, sonorous, high melting and boiling points, good conductors of electricity, good conductors of heat, mainly solids at room temp. This is why it tends to draw electrons.
Nonmetals are elements that form negative ions by gaining electrons during chemical reactions.
Typical nonmetals have a dull, coloured or colourless appearance; Most metals are hard, except sodium. Metals are good conductors of heat and electricity. The obvious answer would be that they are lustrous.
Metals are on the left of the line, in blue.
Are poor conductors of heat and electricity; What are three chemical properties metals? Generally, metals are in a solid state at room. Nonmetals are on the right of the line, in orange.
Many of the elementary nonmetals are gases at room temperature, others are liquids, and others are solids.
All the metals are good conductors of heat and electricity. Au and cu good conductors metallic luster (shine) malleable ductile chemical properties: Chemical combination, properties of metals, nonmetals & noble (inert) gases.