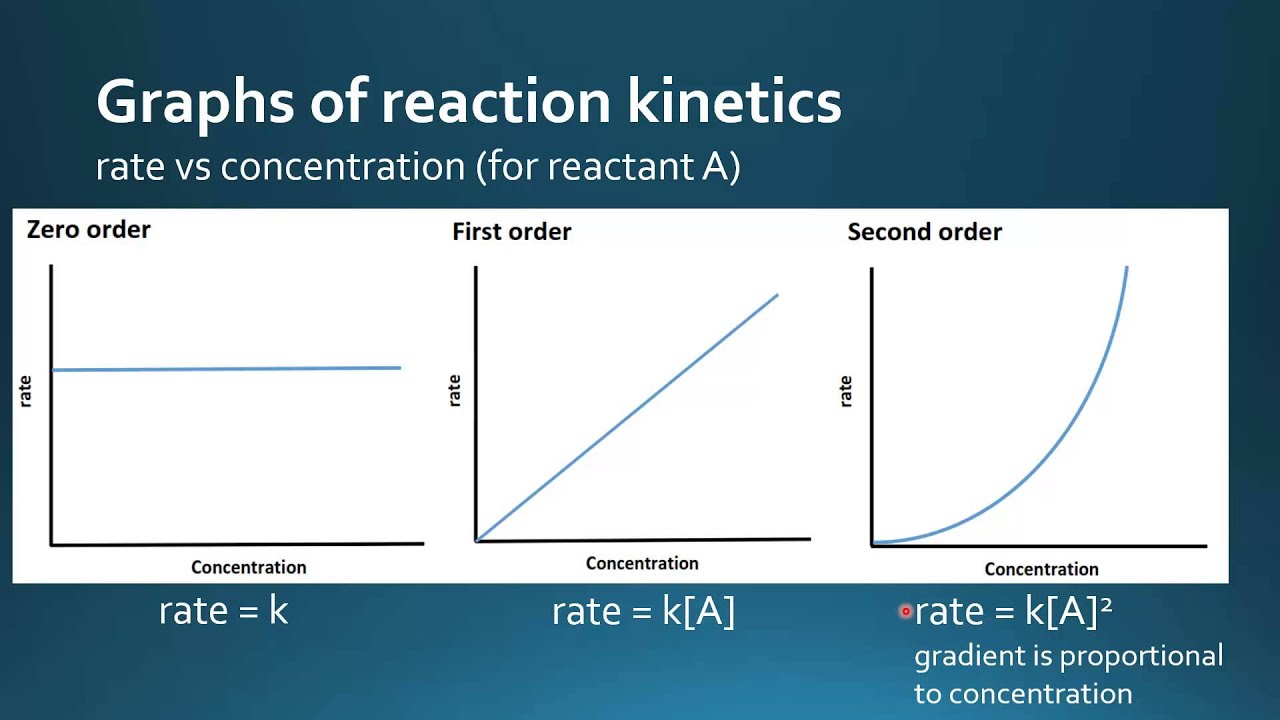
For a zero order reaction: See the answer see the answer done loading. Rate = k[a] rate = k[a] 2:
Integrated Rate Law Zero and Second Order YouTube
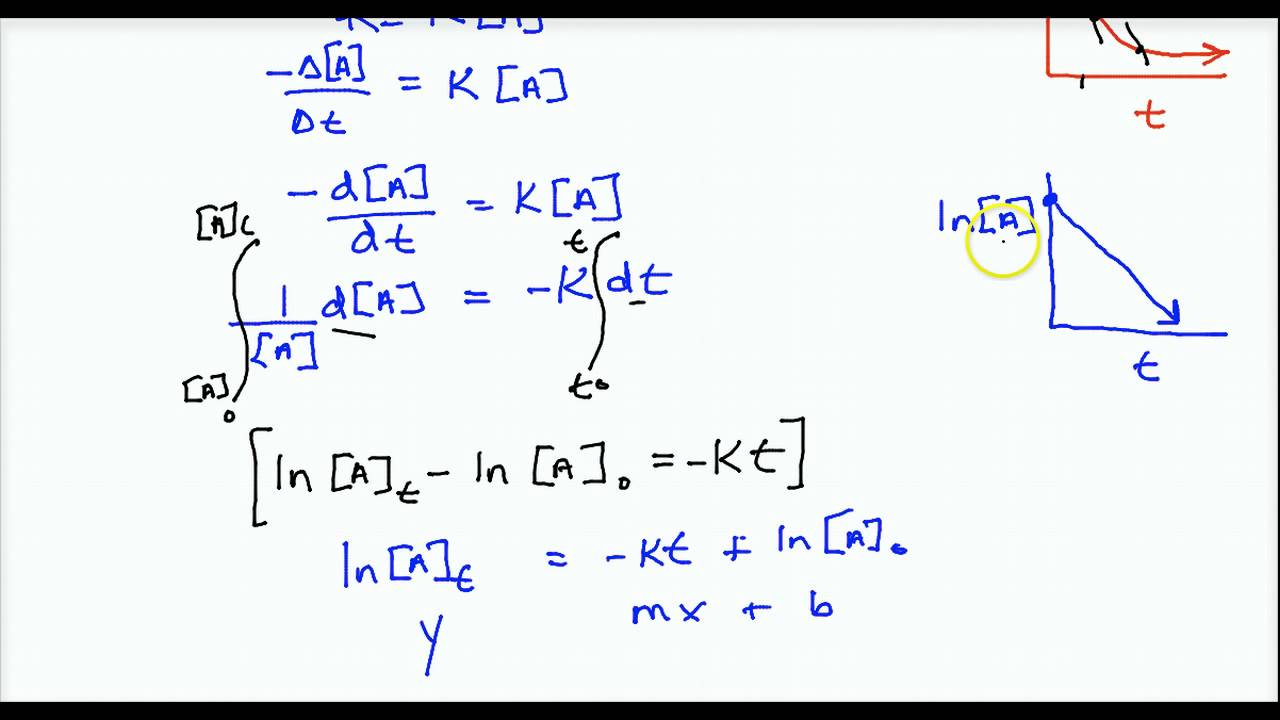
Integrated rate law [a] = −kt + [a] 0:
4 rows this problem has been solved!
This chemistry video tutorial provides a basic introduction into chemical kinetics. Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line. [] [] 0 1 1 x x = + kt • straight line: Substituting the known values into the.
Identify the expression for the rate of the reaction with respect to each of the reactants and products.
2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top. Ln[a] = −kt + ln[a] 0 \(\dfrac{1}{[a]}=kt+\left(\dfrac{1}{[a]_0}\right)\) [] 2 0 1 x = 1 t k 4 note: Rate = k[nh 3] 0 = k.
(k = slope of line) examples.
Rate = k[a] rate = k[a] 2: Therefore, the rate law for this reaction is, rate \( \propto \) [r] Determining k from plots of the integrated rate law expressions is better than using just one pair of [ a] t = − k t + [ a] 0 y = m x + b.
Jay courses 455 view detail preview site
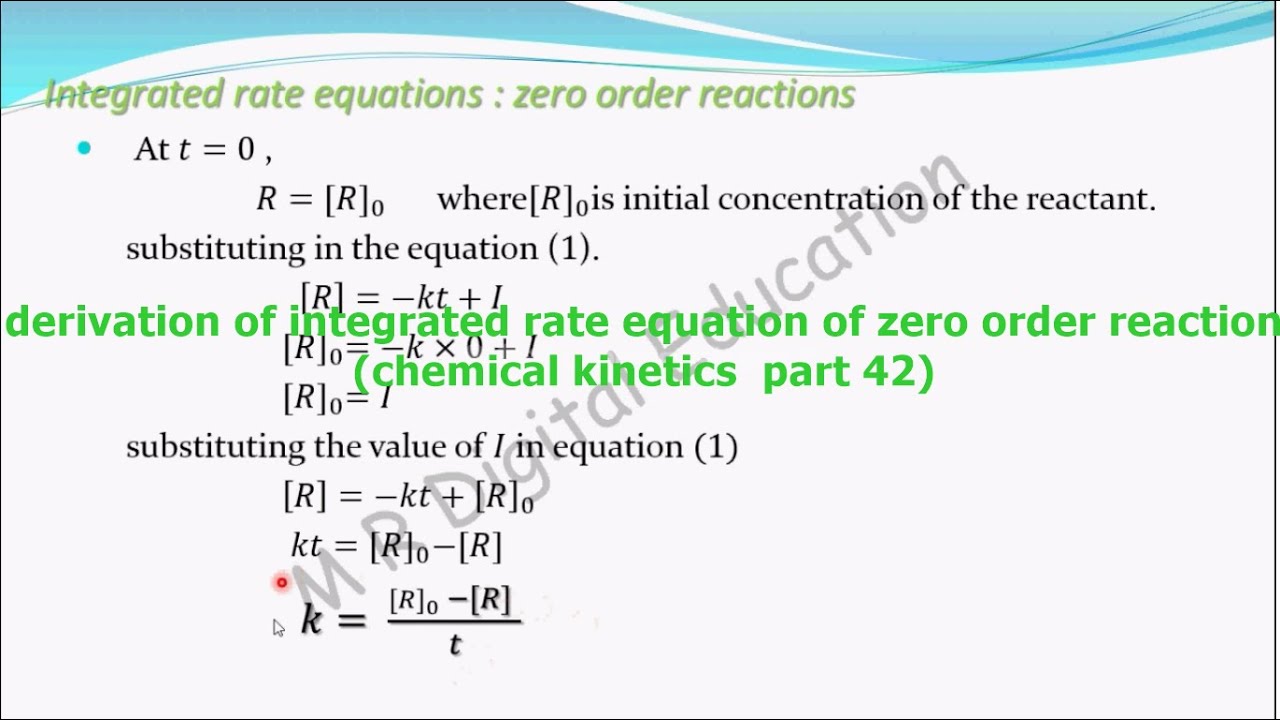
Ln[a] = −kt + ln[a] 0 \(\frac{1}{\left[a\right]}\phantom{\rule{0.1em}{0ex}}=kt+\left(\frac{1}{{\left[a\right]}_{0}}\right)\) In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that is the rate of the reaction is proportional to the first power of the concentration of the reactant. They are used to determine the rate constant and the reaction order from experimental data. The above equation is known as integrated rate equation for zero order reactions.
Integrated rate law [a] = −kt + [a] 0:
[ a] t = − k t + [ a] 0 y = m x + b. Where k is the rate constant plus the natural. Consider the reaction r → p again. Rate = k[a] rate = k[a] 2:
On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed;
A having been given the initial concentration of ethyl chloride ([a] 0) and having the rate constant of k = 1.6 × 10 −6 s −1, we can use the rate law to calculate the concentration of the reactant at a given time t. This video is a lesson on the integrated rate law, which is the equation that determines the dependence of the concentration of a reactant on time.thanks for. The common integrated rate laws. Ln[ ]=− g p+ln[ ]0

.PNG)