We can also determine a second form of each rate law that relates the concentrations of reactants and time. [a] versus t (linear for a zero order reaction) ln [a] versus t (linear for a 1 st order reaction) The above equation is known as integrated rate equation for zero order reactions.
Solved Constants Periodic Table The Integrated Rate Law
Why is it important to know the rate law of a reaction?
Zeroth order (n = 0):
Integrated rate laws are derived from rate laws. We can see that for a zeroth order reaction a plot of [a] vs t will give a straight line, with the. Putting the limits in equation (1) we get the value of c, ⇒ [ a] 0 = c. The equations that relate the concentrations of reactants and the rate constant of.
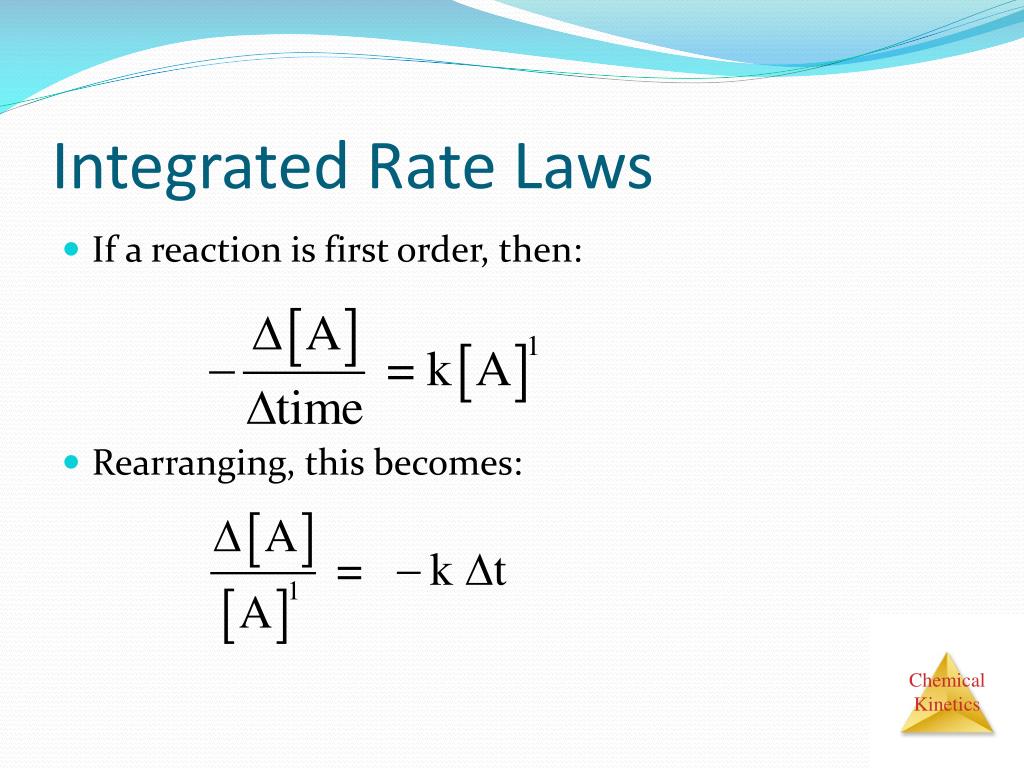
The rate laws we have seen thus far relate the rate and the concentrations of reactants.
To know and be able to apply the integrated rate laws for zeroth, first, and second order rate laws. These are called integrated rate laws. Learners at any stage of their preparation will be benefited by the course. 𝑅 p =− [ ] = g[ ] on the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific.
[a] = − kt + [a]0 y = mx + b.
Where [ a] t is the concentration of a at any time t, [ a] 0 is the initial concentration of. These rate laws help us determine the overall mechanism of reaction (or process) by which the reactants turn into products. The integrated rate law tells you how the concentration of reactant(s) depends on time. At time, t=0, [a] = [ a] 0.
These equations relate reactant concentration with time.
Integrated rate equations integrated rate equations express the concentration of the reactants in a chemical reaction as a function of time. How long does it take for a reactant to reach a specific concentration? Using the integrated rate law expressions, we can find the concentration of a reactant or product present at a particular moment in. Oxalic acid decomposes at high temperature according to the following chemical reaction:
If the initial concentration of the reactant is 0.30 m, how long does it take for the concentration to decrease to 0.15 m?
These are called integrated rate laws. These are called integrated rate laws. We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required. We can also determine a second form of each rate law that relates the concentrations of reactants and time.
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required.
Up to 10% cash back example question #1 : We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the. For reactions of the form aa → products and follow the general rate law rate = k[a]n. It can be noted that the ordinary rate law is a differential rate equation since it offers insight into the instantaneous rate of the reaction.
Rate = k[a]0 = k rate = k [ a] 0 = k.
We can also determine a second form of each rate law that relates the concentrations of reactants and time. The rate laws we have seen thus far relate the rate and the concentrations of reactants. [ a] t = [ a] 0 e−kt. Rate = k[a]0 = k.
The course will be covered in hinglish and the notes will be.
When assessing how quickly a reaction develops, we compare the concentration of a reactant to time. Because using the known rate law , a chemist can work backwards to learn the individual steps and mechanism by which a reaction occurs. [a] −kt + [a]0 y. The rate laws we have seen thus far relate the rate and the concentrations of reactants.
The order of the reaction is “n”.
How long will it take for 80.0% of a sample of c 4 h 8 to decompose? Up to 24% cash back integrated rate laws. In order to create equations that can be used to calculate this information, the rate laws must be integrated over time. Rate laws from graphs of concentration versus time (integrated rate laws) in order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs.