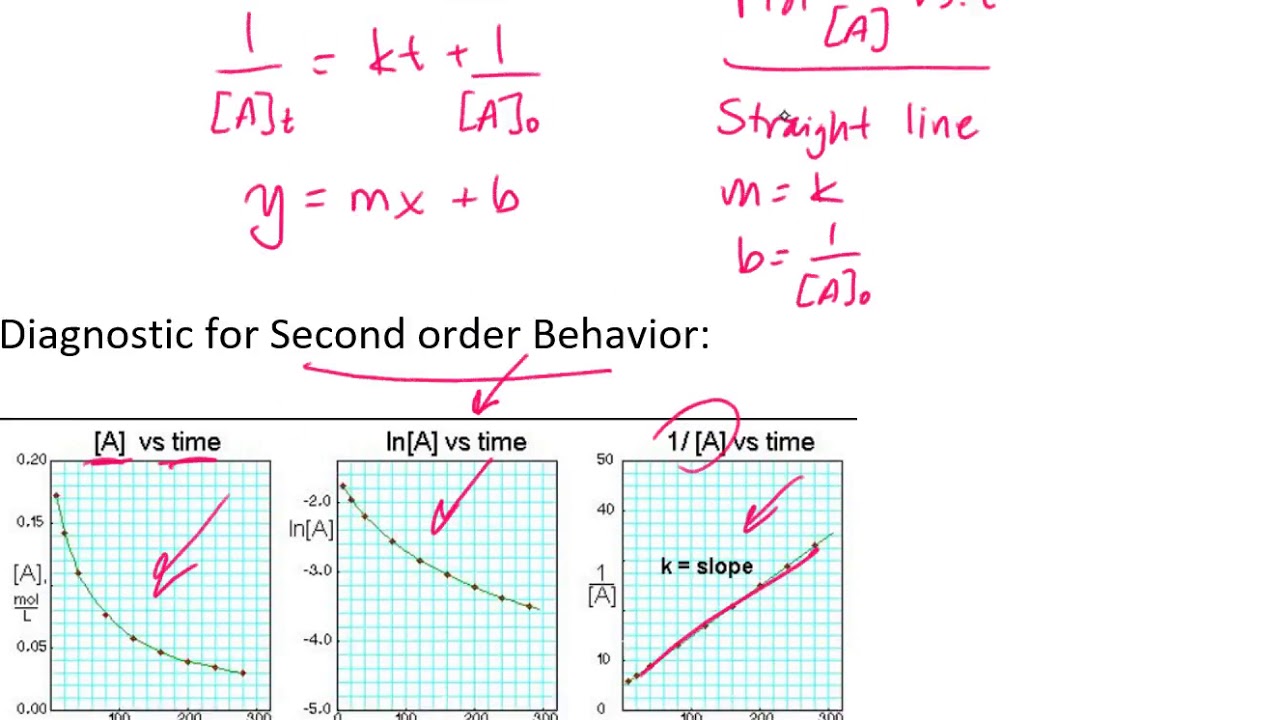
T 1/2 = t 1/2 = t1/2= Relationship between slope of linear plot and rate constant. Setting { \left[ a \right] }_{ 0 } as the initial concentration and [a] as final concentration at given time, t:
AP 14.3 Integrated Rate Laws and Graphing YouTube
At t = 0, [a]t = [a]0, and ln[a]0 = constant.
Reed ap chemistry, p5 12/4/19 chemical kinetics lab date:
For a zero order reaction: They are used to determine the rate constant and the reaction order from experimental data. Rate = k[a]2 r a t e = k [ a] 2. For example, an integrated rate law is used to determine the length of time a radioactive material must be stored.
Which then leads to the integrated rate law you've cited.
This equation is often written as. In the same way, we can derive the integrated rate law for any other order of. Here, [a] is the concentration of reactant “a” and “k” is the rate constant. See the answer see the answer done loading.
Ln[ ]=− g p+ln[ ]0
For zeroth, first and second order, build a chart to organize: 2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top Zero order rate law (integral form) zero order half life zero order rate law first order rate law (integral form) first order half life first order rate law second order rate law (integral form) second order half life second order rate law the science. Rate = k [a]a [b]b [c]c.
This integration gives the integrated rate law.
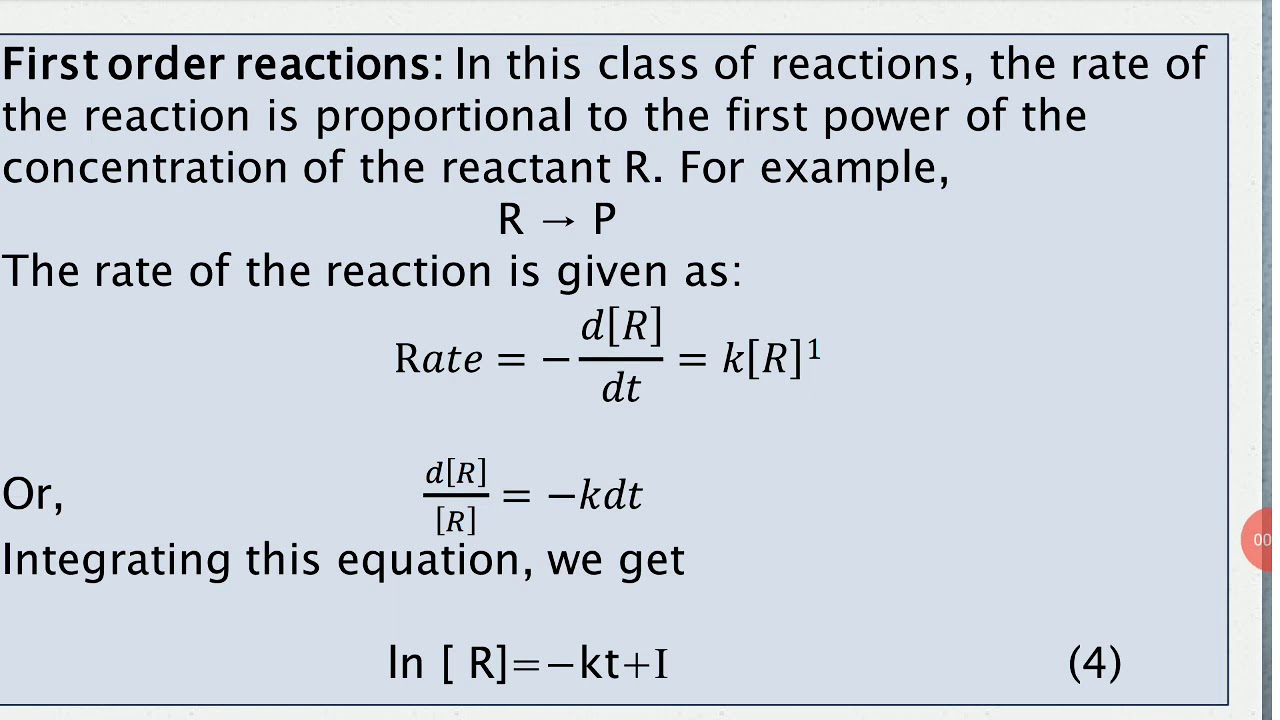
For this type of reaction, the rate law is given as: T is a straight line rate k[a]a 1/alo+kt 1/a] vs. “n” gives the order of reaction. A reaction in which the rate of reaction is proportional to the first power of the concentration of the reactant is called a first order reaction.
Where the exponents, a,b,c,…, may be zero, integers or fractions.
The primary purpose of the integrated rate laws is that they allow us to calculate concentration changes over time. [a] [a]0 = e−kt or. Each equation is specific to its order so the order of a reactant must be known before one can calculate its change in concentration over time. To find the [a] after 122 seconds we use the integrated rate law with the value of k we just found.
Rate law (equation connecting rate and concentration of reactants) rate = rate = rate = units of rate constant.
[a], [a]0, k, and t. The first is that the initial concentrations of a and b are equal, which simplifies things greatly. The sum of the exponents (a+b+c+…) is the order of the reaction. D [ a] d t = − k [ a] 2.
We have a = 2, which leads to the differential equation:
[a] = 0.120 mol/l 4.) the following reaction was studied at a certain temperature, the data in the table were obtained. Similar conventions also apply for 1st and zeroth order kinetics. The rate law calculator has rate of reaction functions for zero order, first order and second order reactions as follows: Plot needed for linear fit of rate data.
So the integrated rate law for a first order reaction is.
In this case, we can say that [a]= [b], and the rate law simplifies to: The differential rate law equation can be integrated to obtain a clear relationship between [a] and time “t”. Ln( [a] [a]0) = − kt or. Determine the rate law, the integrated rate law, the value of the rate constant, and the [h2o2] at 1955 seconds.
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent.
On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed; There are four variables in the equation: