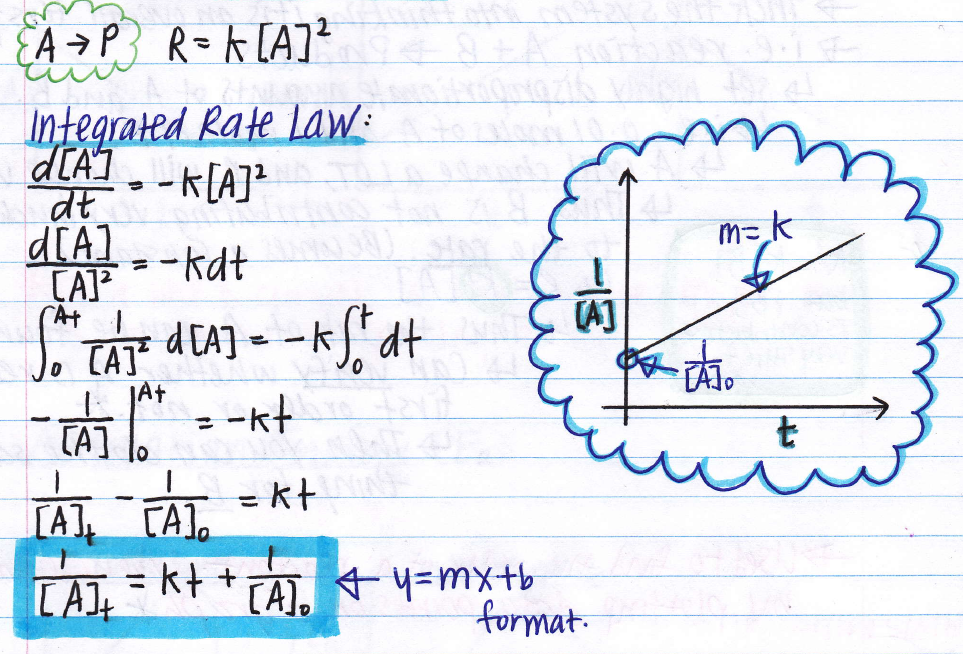
The derivation of the integrated rate laws is generally not required of students of general chemistry (whether high school or college). 1 2 [ ]0=− g p1 ⁄2 +[ ]0; Then, that k can then be used to solve other integrated rate law problems.
Second Order Integrated Rate Law and Half Life (Part 5
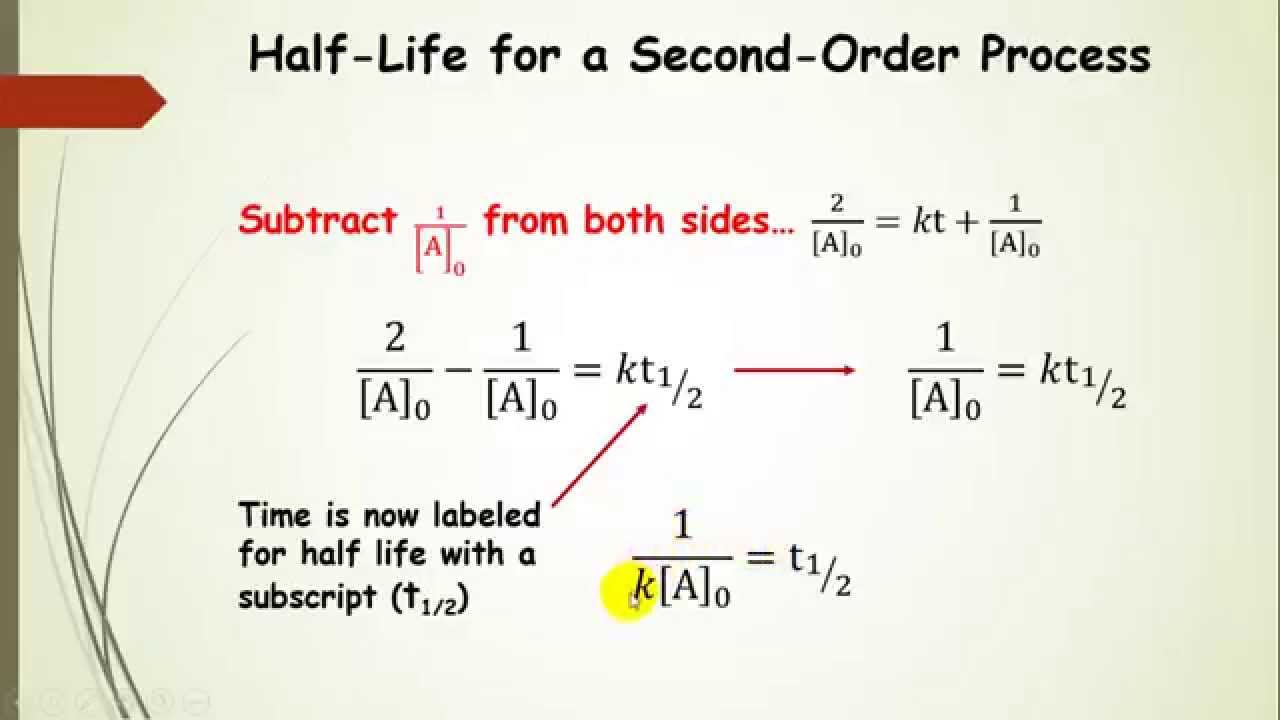
T 1 / 2 = ln [ a ] 0 1 2 [ a ] 0 × 1 k = ln 2 × 1 k = 0.693 × 1 k t 1 / 2 = 0.693 k t 1 / 2 = ln [ a ] 0 1 2 [ a ] 0 × 1 k = ln 2 × 1 k = 0.693 × 1 k t 1 / 2 = 0.693 k
Integrated rate laws are mathematically derived from differential rate laws, and they describe the time dependence of reactant and product concentrations.
Converting a half life to a rate constant; Each integrated rate law can be put into y = mx + b form. A reduction of 50% its original amount). Integrated rate laws express concentration as a function of time.
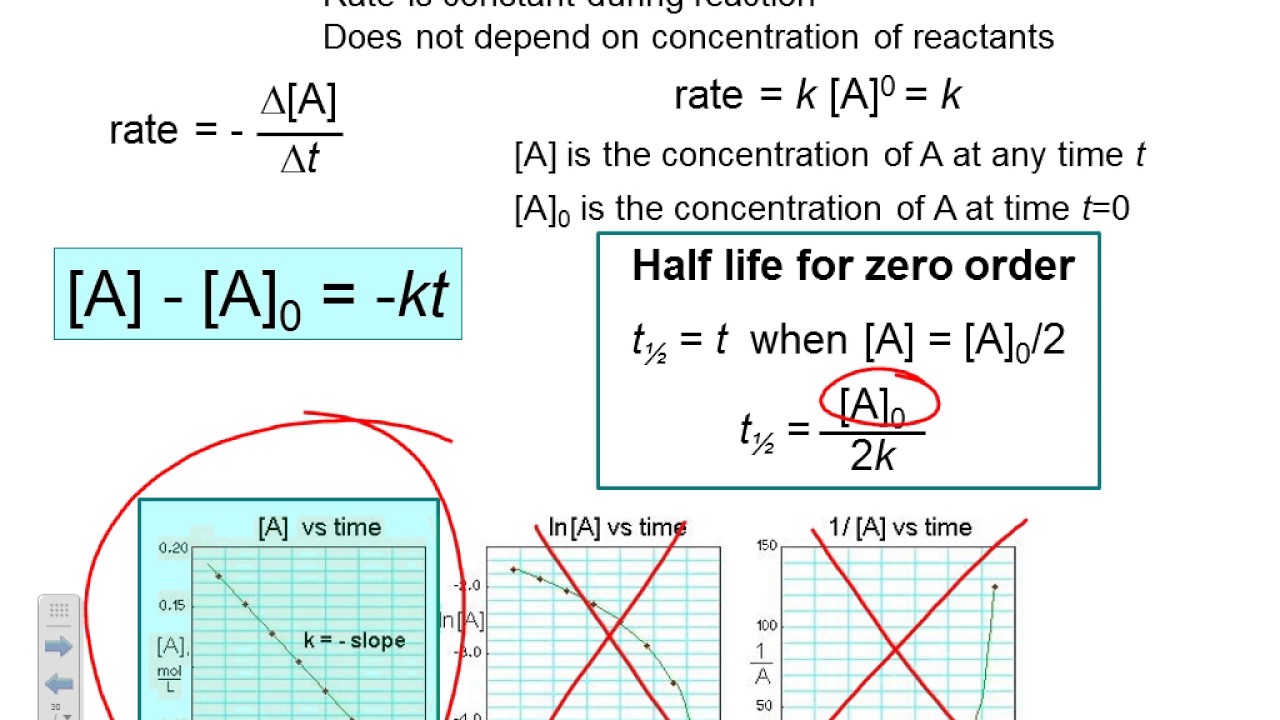
T ½ = [a o] / 2k for a first order reaction a products , rate = k[a]:
Integrated rate law (linear form) 𝐥𝐥𝐥𝐥[𝑨𝑨] = −𝒌𝒌+ 𝐥𝐥𝐥𝐥𝒅𝒅[𝑨𝑨]. T1 / 2 = [a]0 2k. Half life formulas the half life of a reactant is the time it takes until only half of the reactant remains. 7 rows if the plot is a straight line, then the integrated rate law equation can be used to find the.
Graphical relations and half lives;
T ½ = 1 / k [a o] top. 1 [a]t = kt+ 1 [a]0 y = mx+b 1 [ a] t = k t + 1 [ a] 0 y = m x + b. If we replace this idea on the integrated rate law we get: • if presented with a time and concentration data table, before you graph the data to determine
Reactions of orders 0, 1 & 2 have different integrated rate laws.
T ½ = 0.693 / k for a second order reaction 2a products or a + b products (when [a] = [b]), rate = k[a] 2: For a zero order reaction a products , rate = k: