If the plot is not a straight line, then the reaction is not second order. Describe how you will determine the order of a reactant from the data given. Examples of second order reactions.
PPT Chapter 12 Chemical PowerPoint
\(\frac{1}{{[{\rm{r}}]}} = \,{\rm{kt}}\,{\rm{ + }}\,\frac{1}{{{{[{\rm{r}}]}_0}}}\) comparing the above equation with a straight line, \({\rm{y}}\, = \,{\rm{mx}}\,{\rm{ + c}}\) , a plot of the inverse of \({\rm{[r]}}\) as a function of time yields a.
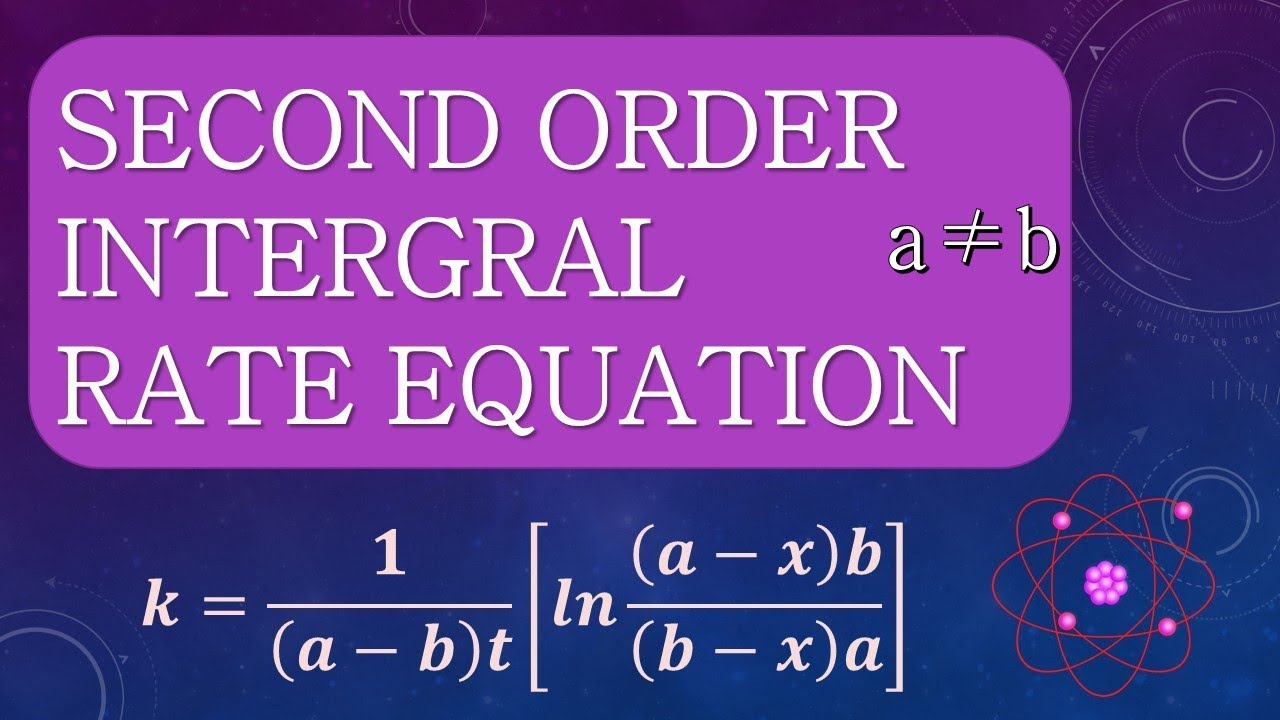
Ln([a]0[b] [b]0[a]) = ([b]0 − [a]0)kt.
Ln( a(b −x) b(a −x)) = (b −a)kt. A few examples of second order reactions are given below: When a reaction is of second order with regard to a specific reactant, an increase in its quantity causes the rate to grow. − d x d t = − k ( [ a] 0 − x) ( [ b] 0 − x)
Click to see full answer.
R = k[a] x [b] y. • use the method of integrated. The integral form of the equation was obtained from the differential form and the full integration can be found here. Where the sum of x and y (which corresponds to the order of the chemical reaction in question) equals two.
The exponents in the rate law must add up to two, so the only rate law that is not second order is:
I'm interested in the second order reaction a + b p. −d[r] /dt = k[r] 2; This rate law works for all values of a ≠ b. Rate=k[a][b]2 given a rate law of rate=k[a], the [a] refers to:
Where [a],[b], and [c] represent the molar concentrations of reactants.
Then the differential rate laws in these two cases are given by differential rate laws: A chemist calls them second order rate laws because the rate is proportional to. Second order reactions ( j=2) the differential form of the rate law is: Let [ a] 0 = a and [ b] 0 = b, then [ a] = a − x and [ b] = b − x.
[] 2 0 1 x = 1 t k 4 note:
We can arrange this to get the integrated rate law: Mass action kinetics is assumed. In the unusual case that a reaction is second order with respect to a single reactant and zeroth order with respect to all other reactants, we can again come up with an integrated rate law. In terms of the original symbols, the rate law becomes.
This reaction proceeds at a rate proportional to the square of the concentration of one reactant,.
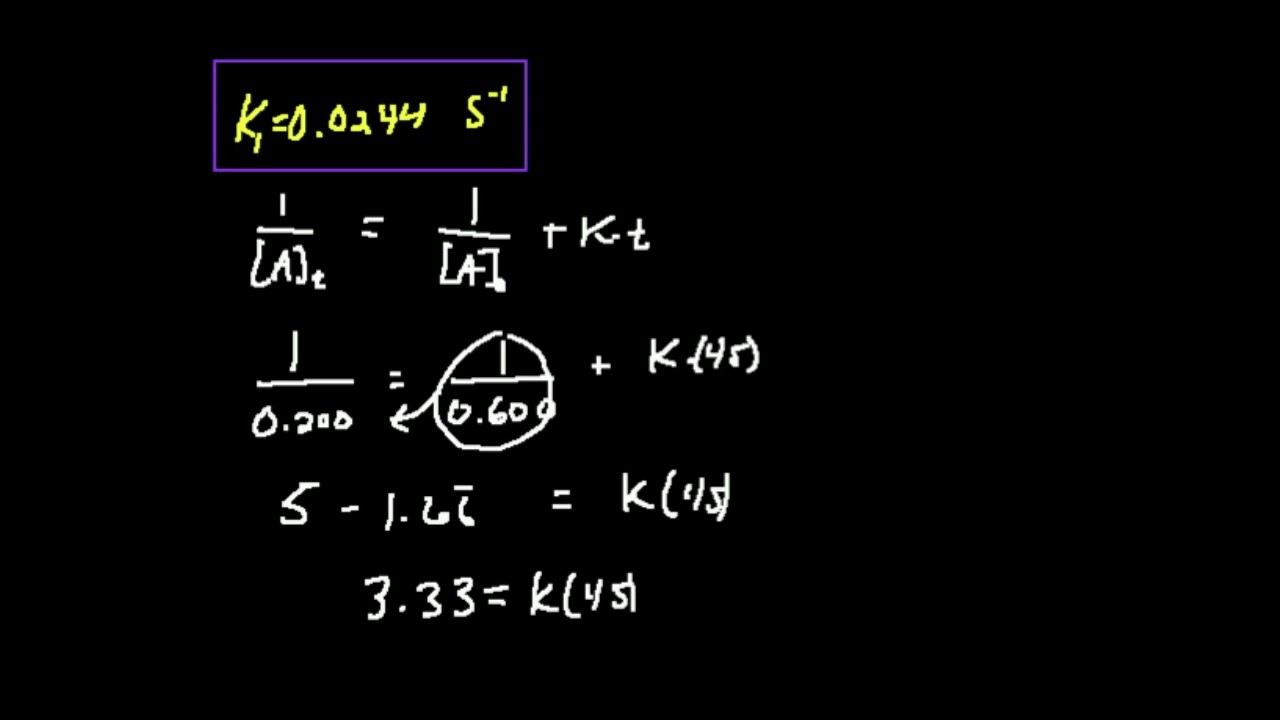
∫ [ ] [ ]2 [𝐴] [𝐴]0 =− g∫ 𝑡 𝑡0 recall from calculus that (or check a table of integrals): This video describes how to obtain the integrated rate law for a second order reaction with two reagents. 1 [ a] t = k t + 1 [ a] 0 y = m x + b. 1 [ a] t = k t + 1 [ a] 0 y = m x + b.
𝑅 p =− [𝑨] 𝒕 = [𝑨] we separate the variables and integrate over the interval:
Since second order reactions can be of the two types described above, the rate of these reactions can be generalized as follows: Write the integrated rate law equations for reactions that are (a) zero order, (b) first order, and (c) second order in [a]. [] [] 0 1 1 x x = + kt • straight line: