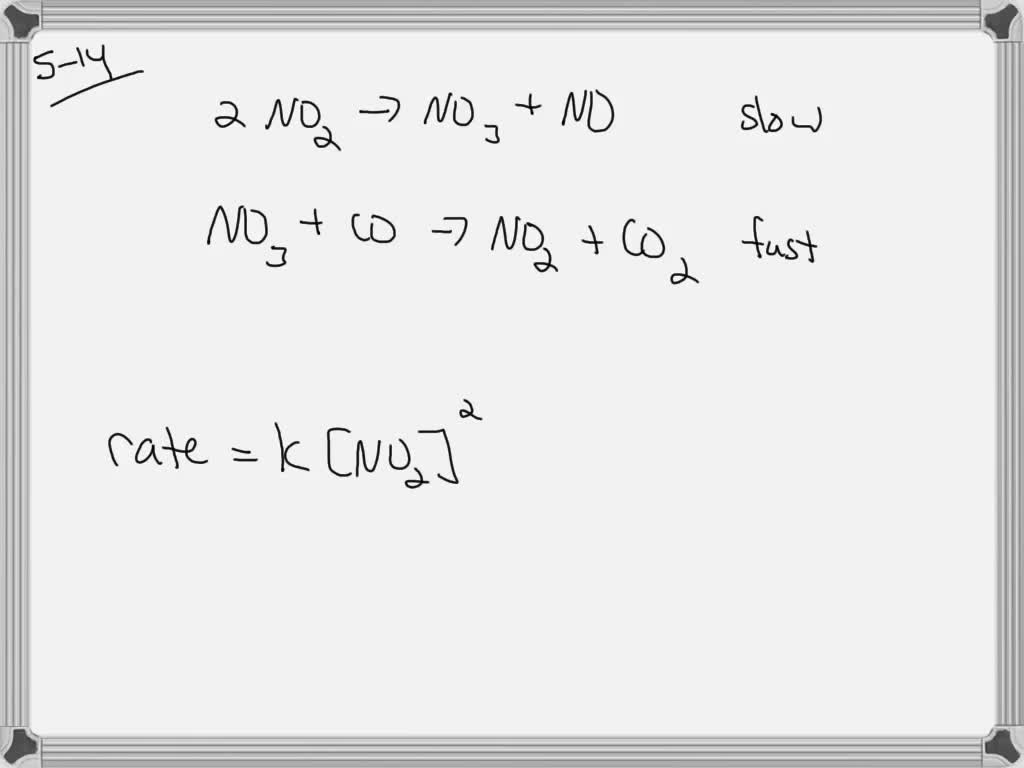In this section, we will look at the integration of 1st, 2nd and 0th order reactions and some interesting graphs that. On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed; How to calculate second order rate constant from graph?
2nd Order Integrated Rate Law Derivation YouTube
Calculate k’ and discuss whether this reaction is kinetically favorable.
[ r] = [ r] 0 2.
The second order integrated rate law is {eq}1/[a]_t = kt + 1/[a]_0 {/eq}. Equations which, in some cases, can be integrated to give a different, but related, form of the rate equation. And, t = t 1 / 2. In mathematical language, these are first order differential equations because they contain the first derivative and no higher derivatives.
When a reaction is of second order with regard to a specific reactant, an increase in its quantity causes the rate to grow.
This means that if i plot the concentration, [a], as a function of time for each expression below, the correct order should yield a straight line function. Since linear graphs resulting from relationships of This curve is defined based on slope of the line, which is approximately equal to a standard. Using the integrated rate law, two products are merged with 1/ [a]_t = 1/ [a]_0 in the event that the last second has no effect.
* products consist of 1/ [a]_t = 1% + 1% + 1%.
By elementary integration of these differential equations integrated rate laws can be. Written by noah january 27, 2022. Ln[ ]=− g p+ln[ ]0 Therefore, the required equation for the half life of.
How to calculate rate constant from second order graph?
The rate law calculator has rate of reaction functions for zero order, first order and second order reactions as follows: Note that the integrated rate law solutions for zero, first and second order expressions are different functions but all can be written in the form of a straight line. Where, [r 0] is the initial concentration of the reactant (when t = 0) [r] is the concentration of the reactant at time ‘t’ k is the rate constant; Determining k from plots of the integrated rate law expressions is better than using just one pair of
Because this equation has the form y = mx + b, a plot of the inverse of [a] as a function of time yields a straight line.
They are used to determine the rate constant and the reaction order from experimental data. Because this equation has the form y = mx + b, a plot of the natural log of [a] as a function of time yields a straight line. Solving integrated rate law problems using the graphing calculator mt. Determine the concentration of hi after 90 days if the initial.
The common integrated rate laws.
The equation for the second order integrated rate law takes the form y = m x + b, where y = 1/a; The differential rate law can be integrated with time to describe the change in concentration of reactants with respect to time. Now, substituting these values in the integral form of the rate equation of second order reactions, we get: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order and rate constant of a reaction.
Therefore, while attempting to calculate the half life of a reaction, the following substitutions must be made:
To use this online calculator for rate constant of first order reaction, enter initial concentration (c0), amount reacted in time t (x) & reaction time (t) and hit the calculate button. The rate constant for the reaction can be determined from the slope of the line, which is equal to k. The integrated rate law can be rearranged to a standard linear equation format: A chemist calls them second order rate laws because the rate is proportional to the product of two concentrations.
2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law.
Using the integrated rate law expressions, we can find the concentration of a reaction or product present after sometime in the reaction. 2nd order reaction integrated rate law 1. Zero order rate law (integral form) zero order half life zero order rate law first order rate law (integral form) first order half life first order rate law second order rate law (integral form) second order half life second order rate law the science. The integral form of the equation was obtained from the differential form and the full integration can be found here.
For a zero order reaction: