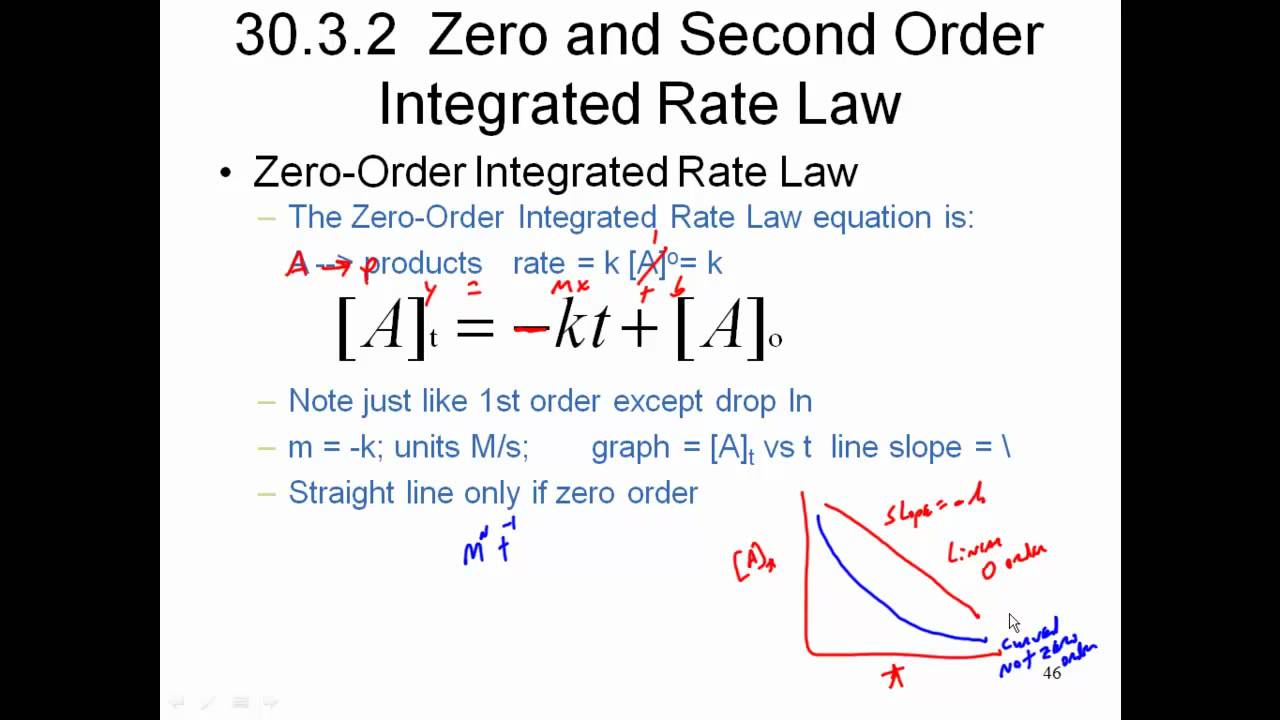
How long does it take? Rate = k[a]0 = k. The integrated rate law equation is a mathematical relationship between the reaction rate and the reactant concentrations.
Chapter 30 HW 10 zero and second order integrated rate
2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top.
Therefore, the expression for integrated rate law for a zero order reaction is $ x = {k_0}t $.
Therefore, the rate law of a zero order reaction would be rate α [r] 0 where [r] is the concentration of the reactant. The above equation is known as integrated rate equation for zero order reactions. Differential and integral form of zero order reaction. Zero order reaction | integrated rate law | half life | jee mains/neet | #shorts #youtubeshorts #shorts #youtube_shorts #youtube #littlestar #youtubevideo #y.
Trelationship between slope of linear plot and rate constantk = −slopek = −slope
The rate law can be written. Rate = k [a] 0. [a] = − kt + [a]0 y = mx + b. This relationship may depend more on the concentration of a single.
(∴ [a] 0 = 1) −d [a] = k dt.
What is the integrated rate law for a zeroth order reaction? That's the expression for integrated rate law for zero order reaction. Always remember the concept that in the zero order reaction, the reaction in which on changing the concentration of reactant, there is no change on the rate of the reaction. Following the mathematical approach of previous examples, the slope of the linear data plot (for decomposition on w) is estimated from the graph.
Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line.
It depends on the dependency of the rate of reaction on the reactants. Derive the integrated rate equation for the rate constant of a. The common integrated rate laws. Haber’s process follows zero order reaction.
Rate laws from graphs of concentration versus time (integrated rate laws) in order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs.
For a zero order reaction: If the rate is independent of the reactants, then the order of reaction is zero. [a] versus t (linear for a zero order reaction) ln [a] versus t (linear for a 1 st order. Decomposition of nh 3 on a hot platinum surface:
Rate = k[a] rate = k[a] 2:
It is of the form y = mx + c.