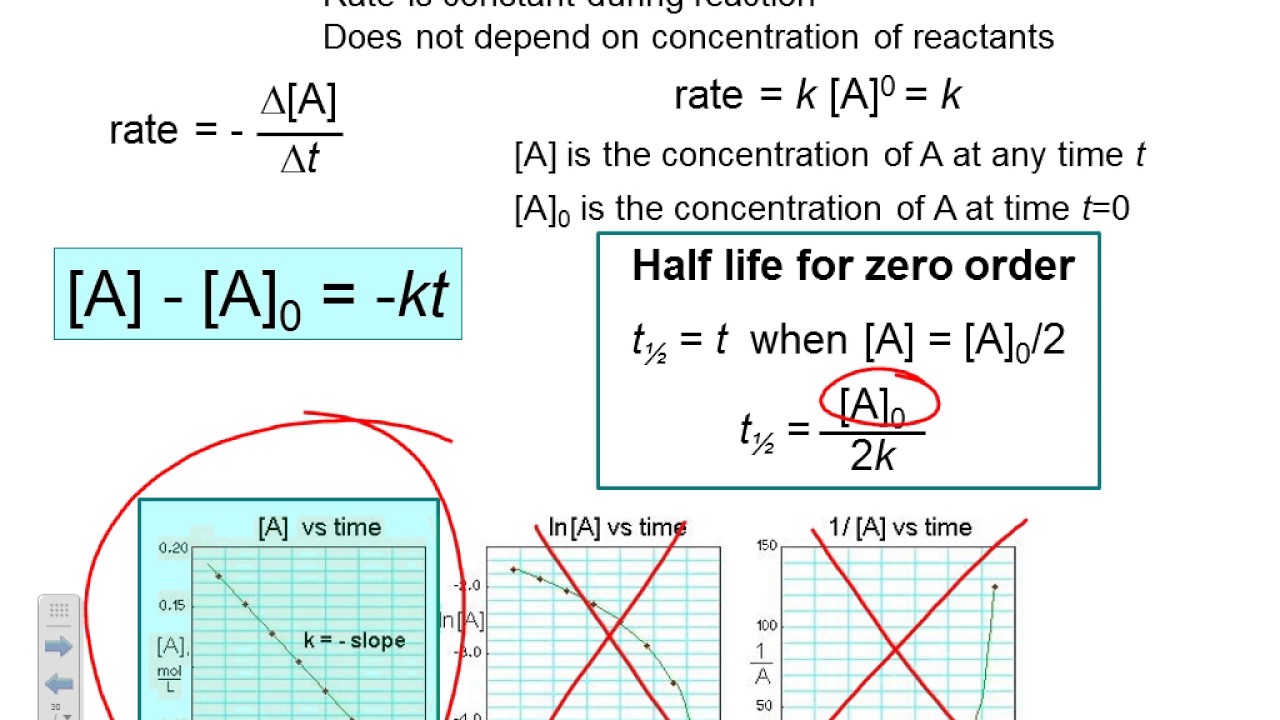
(a) sketch the following plots: [a] = − kt + [a]0 y = mx + b. 𝑅 p =− [𝑨] 𝒕 = [𝑨] = in order to be able to integrate with ease, we can use a technique called separation of variables to get:
Derive Integrated Rate Equation For Constant Of A Zero
(i) rate versus [a] t and (ii) [a] t versus t.
It depends on the dependency of the rate of reaction on the reactants.
Rate = k[a]0 = k. What is the integrated rate law for a zeroth order reaction? Let us derive the integrated rate equation for a 1st order reaction with a rate constant, k. Zero order reaction | integrated rate law | half life | jee mains/neet | #shorts #youtubeshorts #shorts #youtube_shorts #youtube #littlestar #youtubevideo #y.
Therefore, the rate law of a zero order reaction would be rate α [r] 0 where [r] is the concentration of the reactant.
The integrated rate law can be rearranged to a standard linear equation format: Therefore, the expression for integrated rate law for a zero order reaction is $ x = {k_0}t $. If initial concentration of [ a ] is [ a ] 0 then, which of the following statement is incorrect? Always remember the concept that in the zero order reaction, the reaction in which on changing the concentration of reactant, there is no change on the rate of the reaction.
Where k is the rate constant plus the natural.
Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line. Haber’s process follows zero order reaction. For reaction a → b, the rate law expression is − d t d [a] = k [a] 1 / 2. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the negative sign since the reactant disappears):
Trelationship between slope of linear plot and rate constantk = −slopek = −slope
Jay courses 455 view detail preview site If the rate is independent of the reactants, then the order of reaction is zero.


.PNG)


