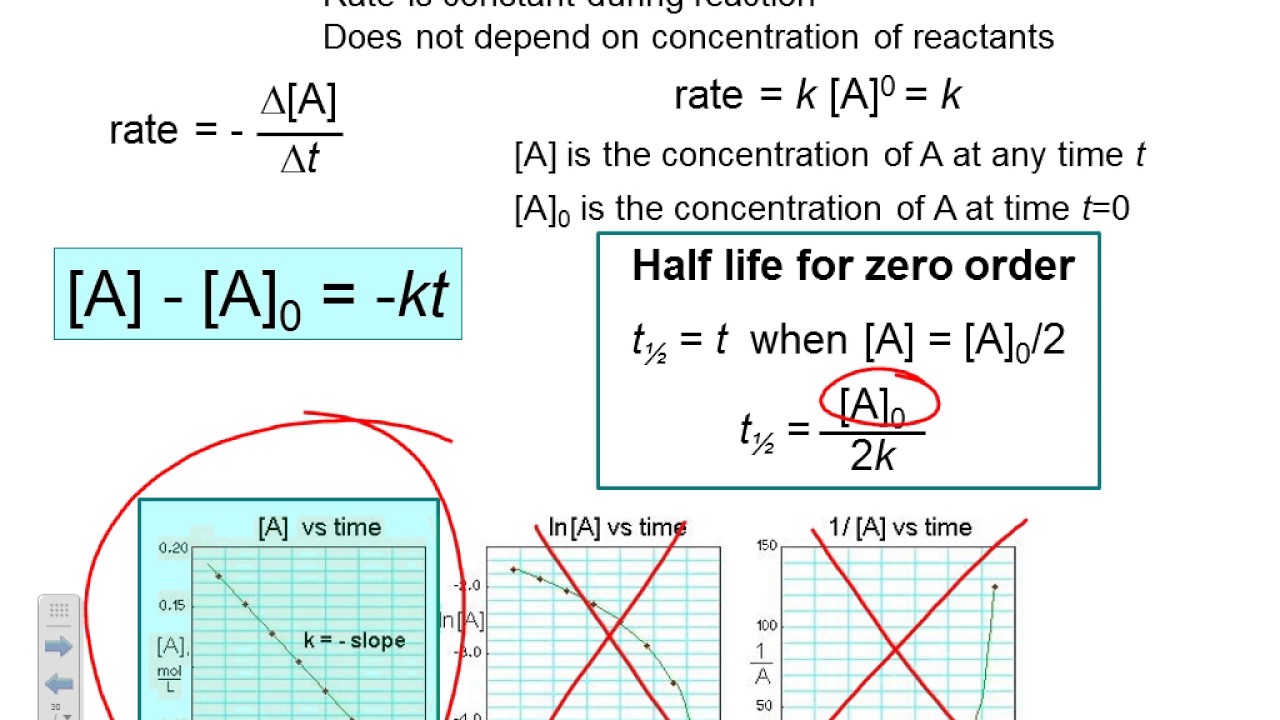
[] [] 0 1 1 x x = + kt • straight line: Graphs of [a], ln[a] or nd i/[a] (y) vs time (x) will give a straight line when the reactions are zero, 1st or 2 order, respectively. First order (n = 1):
integrated second order rate law YouTube
Rate =− d[a] dt = k[a]2 0 d[a] [a]2 = −k dt ⇒ d[a]′ [a][a]′2 0 ∫[a] t =−kdt′ 0 ∫t this leads to:
It is found by looking at the data.
T will be a line with slope k. Consider the reaction a + 2b c + d. The order of the reaction is “n”. Equating the previous equations with each other:
Calculate the value of k, the rate law constant.
The decomposition of hydrogen peroxide in dilute sodium hydroxide solution is described by the equation: If the plot is not a straight line, then the reaction is not second order. A (+ other reactants) →products. Click to see full answer.
They are used to determine the rate constant and the reaction order from experimental data.
On the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific amount of time (t) has passed; = k2t + 1 [a]o. 1 [a] t − 1 [a] 0 = k t or 1 [a] t = k [] 2 0 1 x = 1 t k 4 note:
There are no analytical solutions.
For example rate = k[no 2]2 this reaction is 2nd order in no 2 four factors that affect a reaction rate 1. 1 [ a] t = k t + 1 [ a] 0 y = m x + b. Considering the scenario where one second order reactant forms a given product in a chemical reaction, the differential rate law equation can be written as follows: All rights reserved 31 1 [ ] 2 0 1 = a t k
Up to 20% cash back integrated rate laws worksheet [ ] [a] kt a.
The rate law for this reaction is second order in a and second order in b. These are inherently differential equations, because the rate is always defined as a change in concentration with time; Graphs of [a], ln[a] or Zeroth order (n = 0):
2(g) • write the overall rate expression for this experimentally determined second order reaction.
The rate law for this reaction is first order in a and first order in b. 1 → 2 → (85) where k1 and k2 are the rate constants for the first and second steps, respectively. − d [ r] d t = k [ r] 2. 2h 2o2(aq) → 2h 2o(l) + o 2(g)
K a t a rate [] (2a) [] k a 2 t a rate (2b) 1st order 2nd order solving the differential equations 2a and 2b yields the integrated rate laws:
Second order, one reactant if second order kinetics apply, a plot of 1/[a] vs. For reactions of the form aa → products and follow the general rate law rate = k[a]n. 1 [ a] t = k t + 1 [ a] 0 y = m x + b. Integrated rate law second order 1/[a] = kt + 1/[a] o so if you plot 1/[a] vs time you get a straight line with a slope of k.
Recalling the average rate reaction:
Remember to report your answer with units. The rate constant for the reaction was determined to be. Concentration of reactants this is the rate law. B) a second reaction is run starting with an initial concentration of 0.
In order to obtain the integrated rate equation, this differential form.
1 [a] t − 1 [a] 0 = k t or 1 [a] t = k t + 1 [a] second order, one reactant rate = k[a]2 d[a] [a]2 = −k dt this leads to: Aa → bb second order equation: In other words, a is the