Concentration at any moment of time can be given as, [a] = [ a] 0 e − k t. A full integration of the equation can be found here. Rate = − d a dt d [ a] dt = k [a].
Chem 2 Chemical IV The FirstOrder Integrated
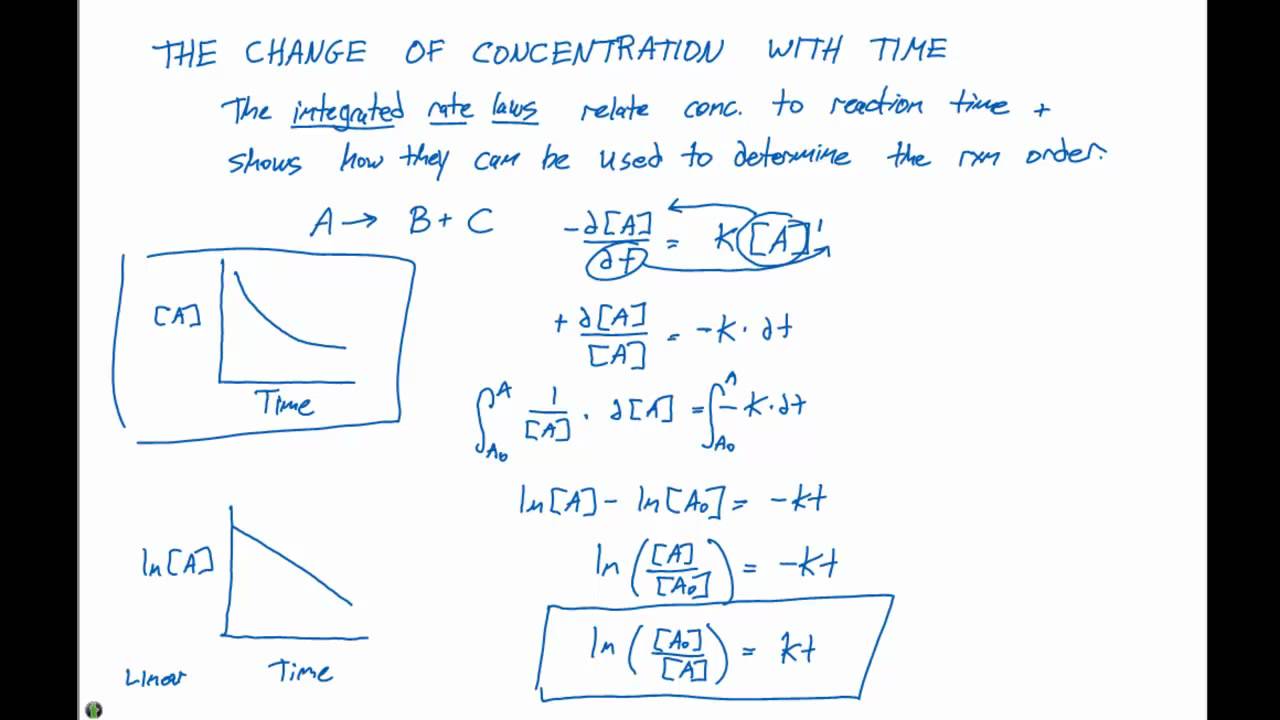
First order reaction is a → product.
Because this equation has the form y = mx + b, a plot of the natural log of [a] as a function of time yields a straight line.
T is the time elapsed since the reaction began; Two typos in the question. This equation (2) is in natural logarithm. The concentration decays from this initial value exponentially as shown below.
Therefore, the rate law for this reaction is,
Where, k is the first order rate constant. The first order rate integral[1] equation calculates the rate at which the reactants turn in to products. (2) let [a] 0 be the initial concentration of the reactant a at time t = 0. For a first order reaction:
The integrated rate law can be rearranged to a standard linear equation format:
Rate = k[nh 3] 0 = k. These are inherently differential equations, because the rate is always defined as a change in concentration with time; (k = slope of line) examples. 2a products or a + b products (when [a] = [b]) , rate = k[a] 2
Integrate the above equation (i) between the limits of time t = 0 and time equal to t, while the concentration varies from initial concentration [a0] to [a] at the later time.
The equation (2) is integrated between limits [a] = [a] 0 at t. Rate law can be expressed as, rate = k [a]1. If the coefficient was different, such as 2a → product, the integrated rate laws would be slightly different. Thus, we can determine the concentration and rate of reaction at any moment with the help of integrated rate equation for zero and first order reaction.
Integration of this ordinary differential equation is elementary, giving:
(1) where, [a] is the concentration of reactant at time t. In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that is the rate of the reaction is proportional to the first power of the concentration of the reactant.