First order reaction is a → product. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The concentration decays from this initial value exponentially as shown below.
integrated rate equation of first order reaction (chemical
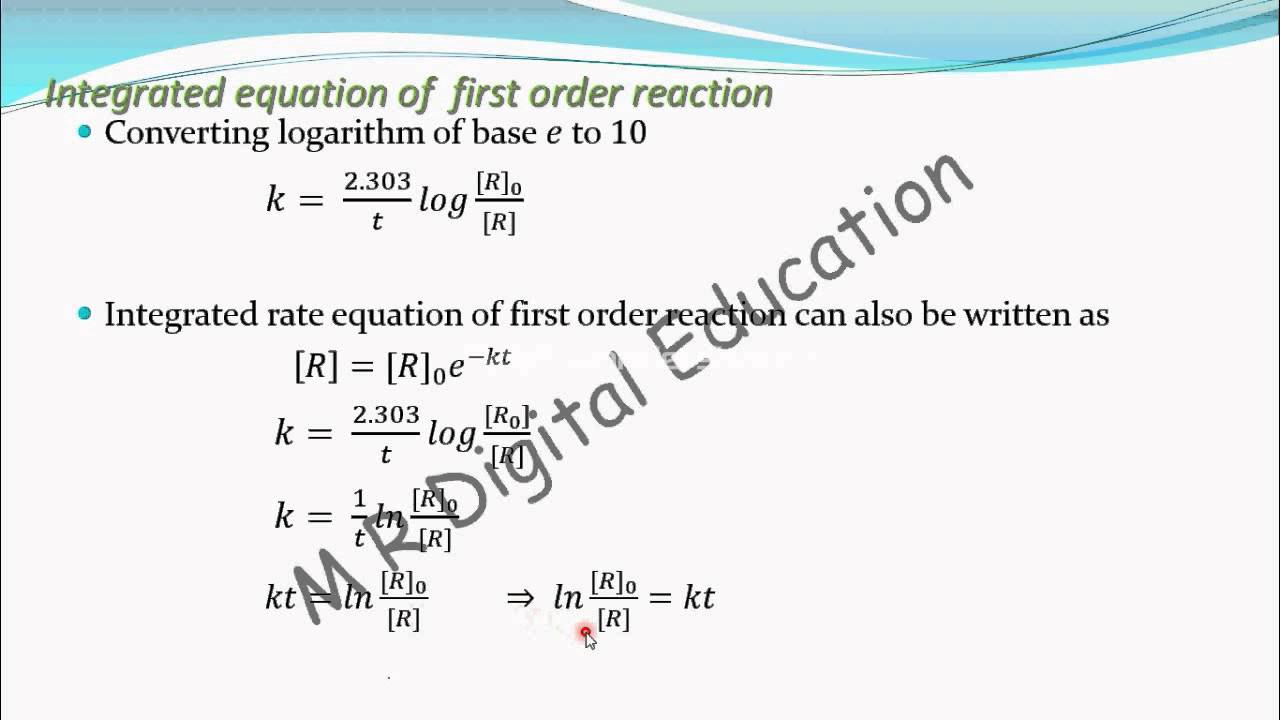
Where k is the rate constant plus the natural.
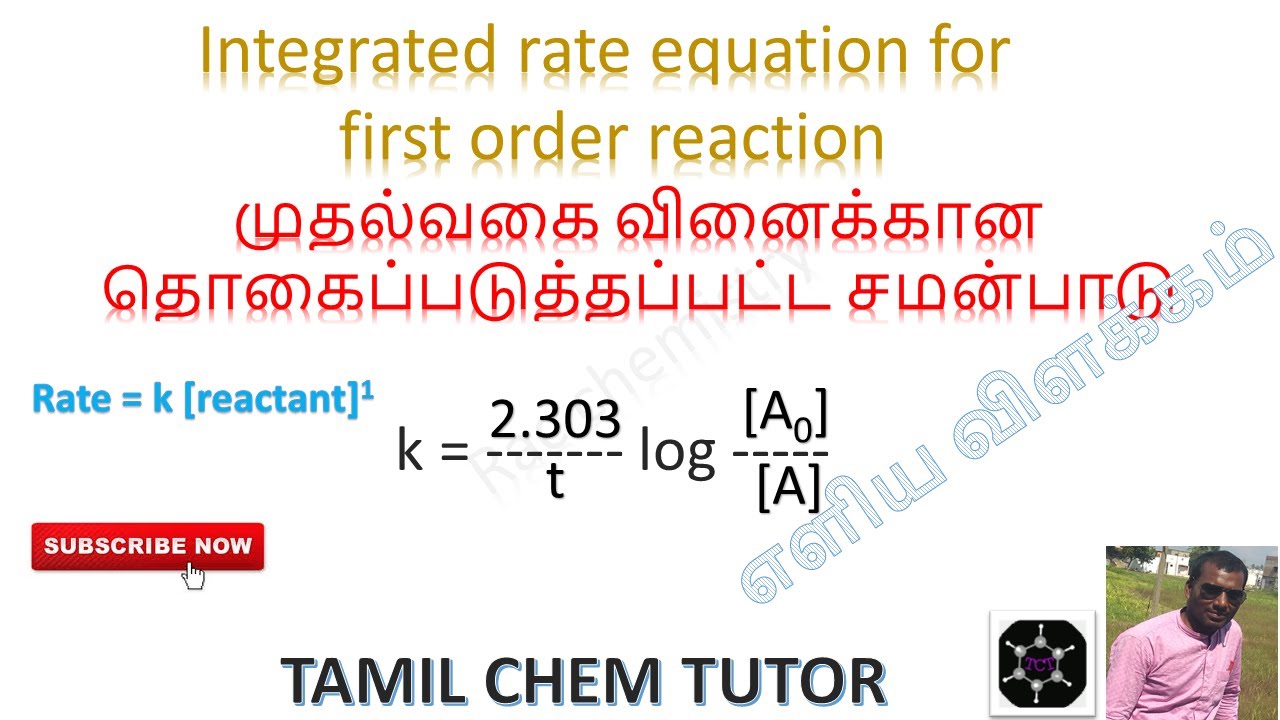
R a t e = k [ a] n.
Jay courses 455 view detail preview site A full integration of the equation can be found here. The differential rate law is given by. From plots of the integrated rate law expressions is better than using just one pair of concentration and rate values, because the plot tends to average out all the experimental errors.
If [ a] = [ b] then, [ a] x + y where x + y = n.
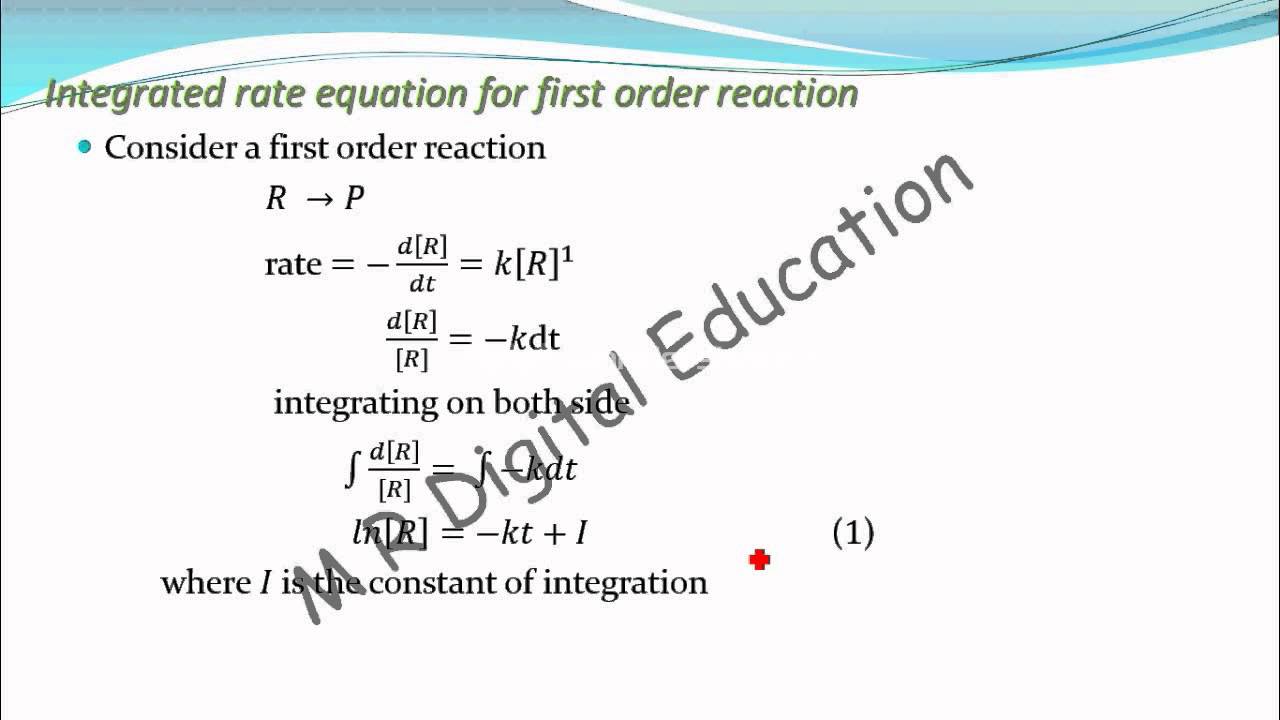
Part a which of these expressions below is the integrated rate law for a first order reaction? In first order reaction, the rate of reaction depends upon the first power of concentration of reactants. For a first order reaction: A general equation for a first order reaction with rate constant k is derived below:
In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that is the rate of the reaction is proportional to the first power of the concentration of the reactant.
Integration of this ordinary differential equation is elementary, giving: Rate law can be expressed as, rate = k [a]1. 4 rows k = r a t e [ a] x [ b] y. Consider first order reaction, a → b + c.
What is the integrated rate law for a first order reaction?
2a products or a + b products (when [a] = [b]) , rate = k[a] 2 Rate = −`(d[a])/dt` = k[a].(1) where, [a] is the concentration of reactant at time t. Integrate the above equation (i) between the limits of time t = 0 and time equal to t, while the concentration varies from. A reaction whose rate depends on the reactant concentration raised to the first power is called a first order reaction.
O rate = k [a]m [b] 1/a=kt + 1/a).
(k = slope of line) examples. The integrated rate law can be rearranged to a standard linear equation format: The equations which are obtained by integrating the differential rate laws and which gives the direct relationship between the concentrations of the reactants and time is called integrated rate laws. The first order rate integral[1] equation calculates the rate at which the reactants turn in to products.
Integrated rate law for first order reaction:
Where, k is the first order rate constant. Consider the reaction r → p again. Natural and artificial radioactive decay of unstable nuclei are examples of first order reaction. Therefore, the rate law for this reaction is,