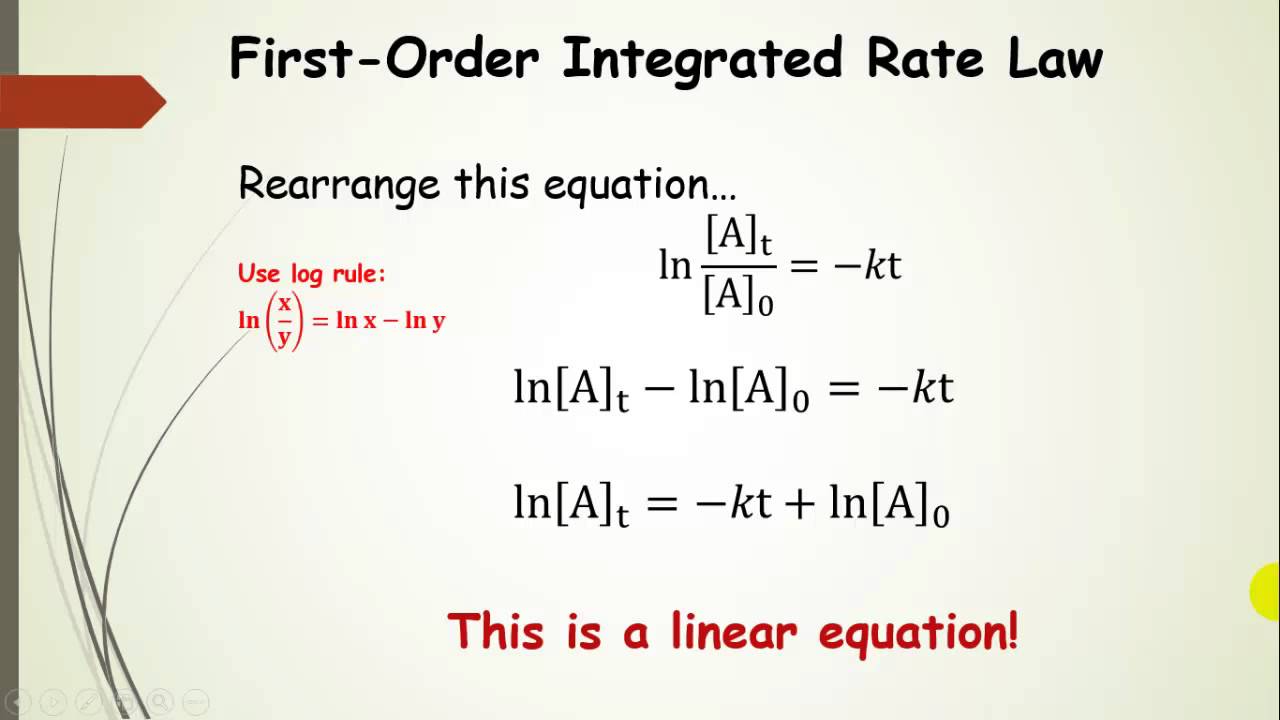
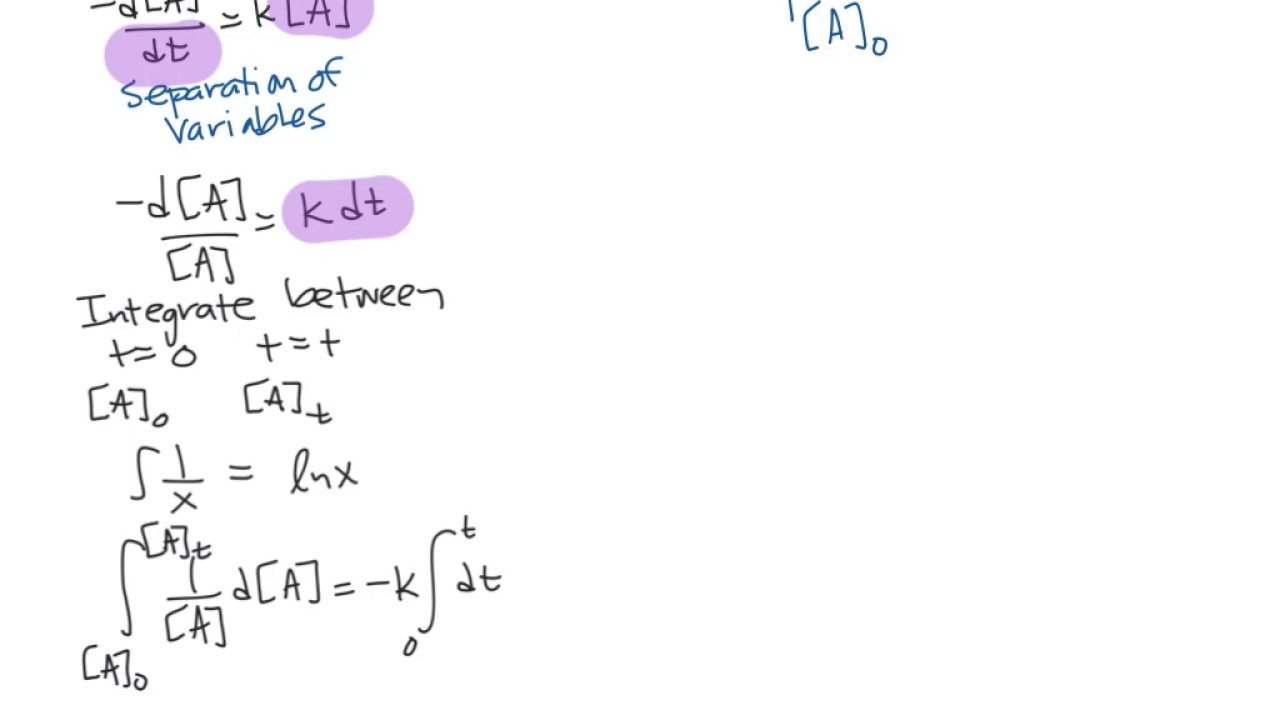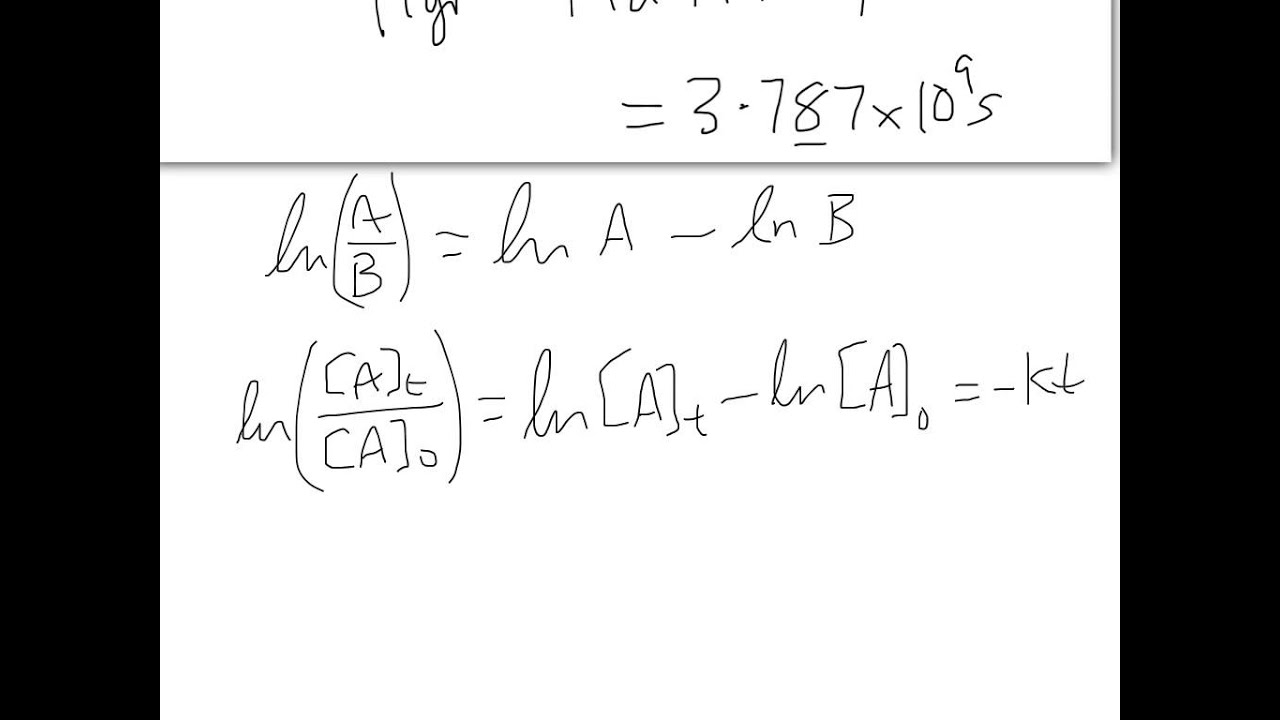1 [ a] t = k t + 1 [ a] 0 y = m x + b. The slope of the straight line signifies the value of rate constant, k. An integrated rate law comes from an ordinary rate law.
Principles of Chem 2
𝑅 p =− [ ] = g[ ] on the other hand, integrated rate laws express the reaction rate as a function of the initial concentration and a measured (actual) concentration of one or more reactants after a specific.
However, integrated rate laws are.
The order of the differential rate equation, of course, determines the form of the integrated equation. The rate laws below are given for a simple general reaction. A = 8n time dependence of n (analogous to integrated rate law): Assume initial 1 l solution and 1 m concentration.
Rate laws from graphs of concentration versus time (integrated rate laws) in order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs.
The rate law or rate equation for a chemical reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). The rate law calculator has rate of reaction functions for zero order, first order and second order reactions as follows: The instantaneous rate of a reaction is given by differential rate law equations. Zero order rate law (integral form) zero order half life zero order rate law first order rate law (integral form) first order half life first order rate law second order rate law (integral form) second order half life second order rate law the science.
The integrated rate law is an equation that describes the concentration of a reactant over time (t).
The above equation is known as integrated rate equation for zero order reactions. We know that the rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may or may not be equal to the stoichiometric coefficient of the reacting species in a balanced chemical equation. Substitute this information into the integrated rate law for a reaction with this order and solve the equation for [a o]. C 4 h 8 2c 2 h 4.
These rate laws help us determine the overall mechanism of reaction (or process) by which the reactants turn into products.
The integrated rate law comes in different forms depending on the reaction order. The reaction is in second order. The differential rate law can be integrated with time to describe the change in concentration of reactants with respect to time. Integrated rate law equation the integrated rate law equation is a mathematical relationship between the reaction rate and the reactant concentrations.
Consider the first order reaction a → products the rate law is:
A plot of ln nt vs. An integrated rate law is an equation that expresses the concentrations of reactants or products as a function of time. This relationship may depend more on the concentration of a single reactant, whereas the rate law results may include none, some, or all of the reactant species involved in the reaction. [a] versus t (linear for a zero order reaction) ln [a] versus t (linear for a 1 st order.
For the reaction given by 2no + o 2 → 2no 2, the rate equation is:
How long (in s) will it take for 80.0% of the reactant to decompose? Important terms and equations for radioactive decay equations radioactive decay law (analogous to differential rate law): 1 [a] = kt + 1 [a]0 y = mx + b. For the chemical reaction a → b + c, integrated rate law can be expressed as a mathematical expression as given below.
The rate constant, k, for the reaction or enough information to determine it.
Here, [a] 0 is the initial concentration of the reactant a and [a] is the concentration of reactant “a” after “t” time has passed. Using the integrated rate law expressions, we can find the concentration of a reaction or product present after sometime in the reaction. 1 [ a] t = k t + 1 [ a] 0 y = m x + b. Nt = no(½) h and a t = ao(½) h t½ = 0.693/8
In this section, we will look at the integration of 1st, 2nd and 0th order reactions and some interesting graphs that.
80% decomposed means new concentration is 0.2 m. For many reactions, the initial rate is given by a power law such as = [] [] where [a] and [b] express the concentration of the species a and. Rate = k[no] 2 [o 2] find the overall order of the reaction and the units of the rate. See what is the rate law?.
The integrated rate laws are given above.
The equation for the second order integrated rate law takes the form y = m x + b, where y = 1/a;