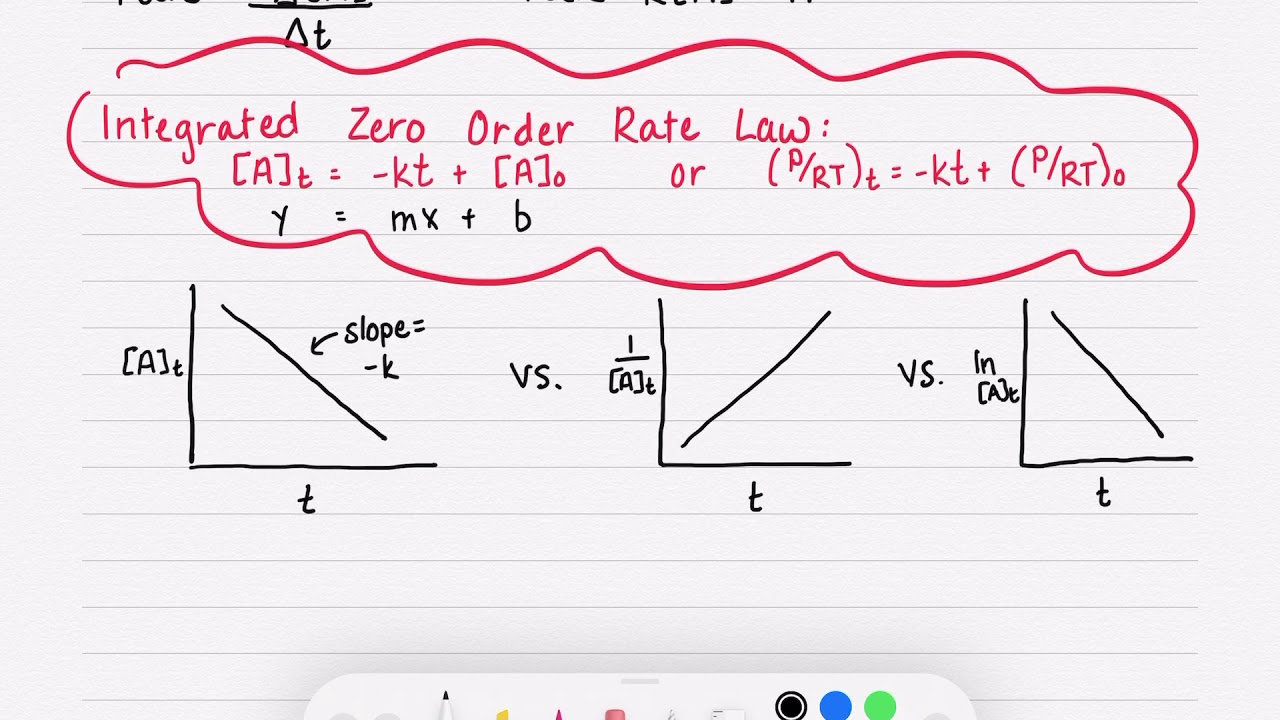
Integrated rate law equation for zero order reaction | class 12th chemistry Because this equation has the form y = mx + b, a plot of the concentration of a as a function of time yields a straight line. The rate law can be written.
graph of integrated rate equation of zero order reaction
It depends on the dependency of the rate of reaction on the reactants.
We get the following when we use the resultant value of c in equation (1):
The common integrated rate laws. (∴ [a] 0 = 1) −d [a] = k dt. Where, [r 0] is the initial concentration of the reactant (when t = 0) [r] is the concentration of the reactant at time ‘t’ k is the rate constant; A reaction is said to be of zero order, if its rate is independent of the concentration of the reactants.
Initial concentration [a] = [a] o at t = 0.
Zero order reaction | integrated rate law | half life | jee mains/neet | #shorts #youtubeshorts #shorts #youtube_shorts #youtube #littlestar #youtubevideo #y. Therefore, the rate law of a zero order reaction would be rate α [r] 0 where [r] is the concentration of the reactant. Consider the general zero order reaction: [a] = − kt + [a]0 y = mx + b.
The differential rate law equation is :
For a zero order reaction: C = constant of integration. Dx/dt = k[a] a [b] b. Rate = k [a] 0.
[ a] t = − k t + [ a] 0 y = m x + b.
The differential form of rate law is transformed to integrated form of rate law by simple mathematics (calculus). [ a] t = − k t + [ a] 0 y = m x + b. 2a products or a + b products (when [a] = [b]) , rate = k[a] 2 the integrated rate law is 1/[a] = kt + 1/[a o] top. Rate = k[a]0 = k.
The above equation is known as integrated rate equation for zero order reactions.



.PNG)

