(2) a + b þ products rate of reaction = k[a][b] integrated rate law very complex as two concentrations are changing simultaneously. (1) where, [a] is the. Consider first order reaction, a → b + c.
PPT Rate Laws PowerPoint Presentation ID4206487
If [ a] = [ b] then, [ a] x + y where x + y = n.
The rate of disappearance reactant of a in the given reaction can be given by the following relation.
A full integration of the equation can be found here. Thus, we can determine the concentration and rate of reaction at any moment with the help of integrated rate equation for zero and first order reaction. Concentration at any moment of time can be given as, [a] = [ a] 0 e − k t. Rate = − d a dt d [ a] dt = k [a].
Using calculus, the rate law can be integrated to obtain an integrated rate equation that links concentrations of reactants or products with time directly.
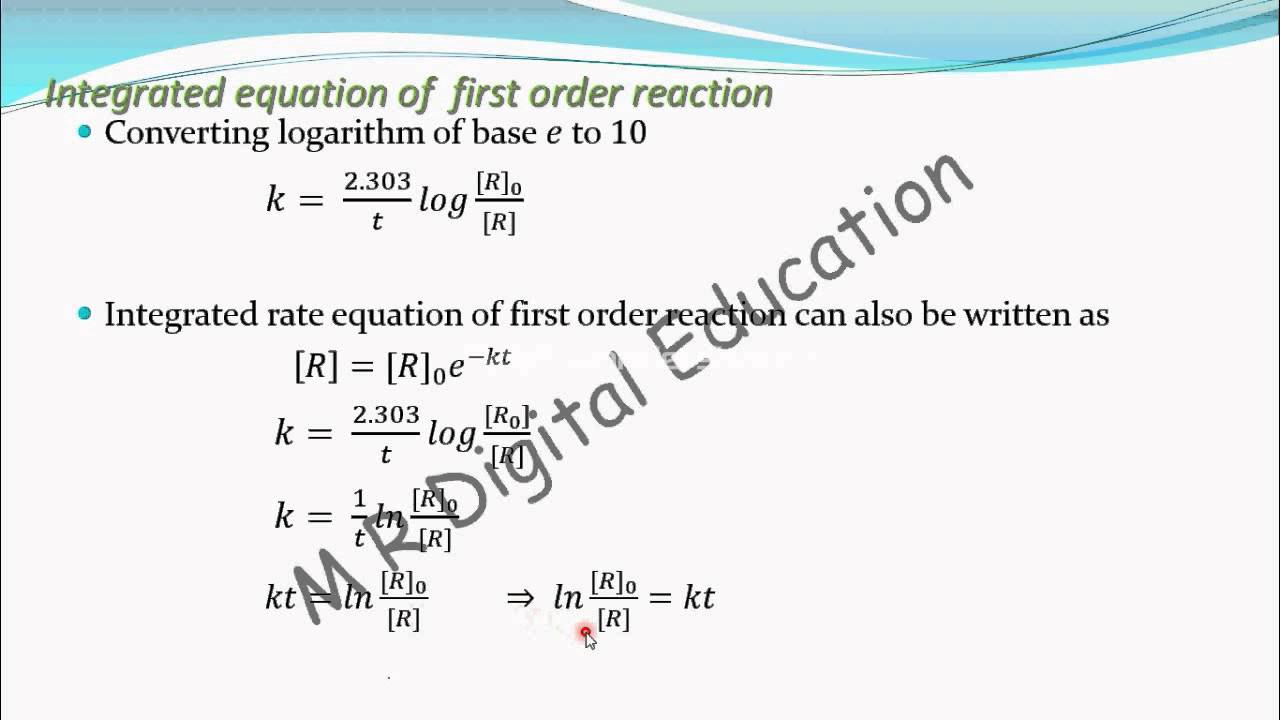
The first order rate integral[1] equation calculates the rate at which the reactants turn in to products. Because this equation has the form y = mx + b, a plot of the natural log of [a] as a function of time yields a straight line. This is an expression for the integrated rate constant for the first order reaction. Therefore, the rate law of this reaction is, rate [r] 0.
At t = 0, the concentration of the reactant r = [r] 0.
(i) where i is the constant if integration. R a t e = k [ a] n. The integrated rate law can be rearranged to a standard linear equation format: Where k is the rate constant plus the natural.
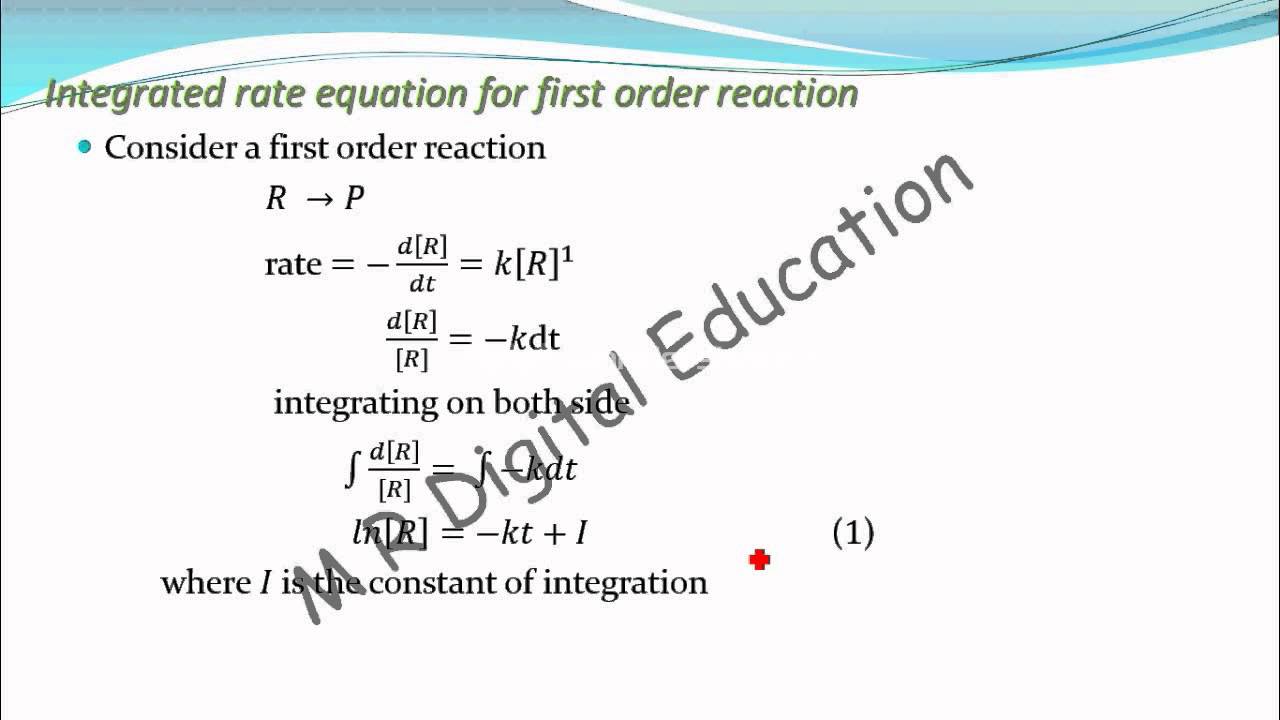
A) consider a general first order reaction r → p the differential rate equation for given reaction can be written as rate = − d t d [r] = k [r] 1 rearrange above equation.
Jay courses 455 view detail preview site (i.e at t = t). − [ ] = 1 (87) or − [ ] = 1 (88) integrating both sides, we get −ln = 1 +i (89) where i is the constant of integration. (1) where i is integration constant
The differential rate law is given by.
K = r a t e [ a] x [ b] y. Integrate the above equation (i) between the limits of time t = 0 and time equal to t, while the concentration varies from initial concentration [a 0] to [a] at the later time. Namely, the concentration versus timedata are fit to the following equation: Where, k is the first order rate constant.
First order reaction is a → product.
This equation (2) is in natural logarithm. The expression for the integrated rate constant in terms of initial concentration for first order reaction: Integrated rate law equation for first order reaction | class 12th chemistry [r] d [r] = − k × d t integrating on both sides of the given equation ∫ [r] d [r] = − k ∫ d t ln[r] = − k t + i.