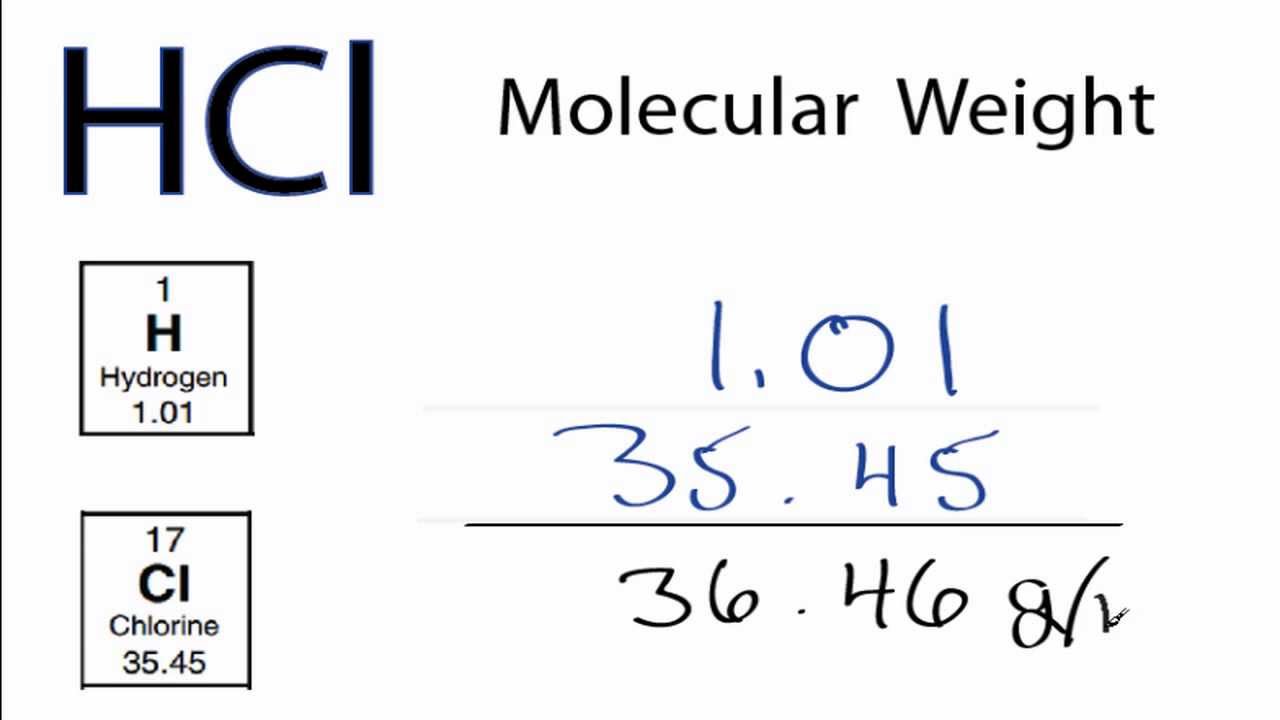Nitric acid produces nitrate salts. What is produced when magnesium oxide reacts with hydrochloric acid? Complete lonic equation net lonic equation:
Hydrochloric Acid Solution, Sequencing Grade
Hydrochloric acid has many uses.
8 rows both hydrogen chloride and hydrochloric acid are corrosive.
This is the word equation for my investigation, calcium + hydrochloric calcium + carbon + water [image]carbonate acid chloride dioxide. For example, hydrochloric acid has the formula hcl, sulfuric acid is h₂so₄ and nitric acid as shown in the example is hno₃. What mass of hydrochloric acid reacts with 28.05 g of calcium oxide? Lead (ii) nitrate (aq) + potassium chromate (aq) observation:
Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals.
For math, science, nutrition, history, geography, engineering, mathematics. What salt is produced by hydrochloric acid? Hydrochloric acid + zinc carbonate arrow zinc chloride + carbon dioxide + water 2hcl(aq) + znco3(s) arrow zncl2(aq) + co2(g) + h2o(l) Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) explanation.
Click to rate this post!
Magnesium reacts with hydrochloric acid according to the equation: Of atoms of each element in reactants is equal to no. What is the word equation for magnesium oxide and hydrochloric acid? The chemical formula of hydrochloric acid is \({\text{hcl}}{\text{.}}\) hydrochloric acid is the aqueous solution of hydrogen chloride.
Cao + 2hcl → cacl 2 + h 2 o.
The balanced chemical equation is: Complete ionic equation net lonic equation: What mass of calcium chloride is formed when 28.05 g of calcium oxide reacts with excess hydrochloric acid? The symbol equation for my investigation is, [image]caco3 + 2hcl cacl2 + co2+ h2o
Hcl + naoh = nacl + h2o
Hydrochloric acid produces chloride salts. Balanced chemical equation of caco 3 and hcl reaction with physical states caco 3(s) + 2hcl (aq) → cacl 2(aq) + co 2(g) + h 2 o (l) calcium carbonate is not soluble in water and exists as white precipitate in the water. Calcium oxide reacts with hydrochloric acid according to the equation: Sodium sulfide (aq) + hydrochloric acid (aq) observation:
Extended keyboard examples upload random.
When aqueous hydrochloric acid is added, calcium chloride, carbon dioxide and water are formed. I am investigating how the rate of reaction between marble chips and hydrochloric acid is altered when the concentration is changed. Therefore, we use the formula \({\text{hcl}}\) for both hydrochloric acid and hydrogen chloride. Of atoms of each element of products.
A balanced symbol equation for sodium hydroxide with hydrochloric acid?
Cu + hcl (copper + hydrochloric acid): Acids always have hydrogen (h) in them. This reaction is an example of a single replacement. Sodium hydroxide + hydrochloric acid → sodium chloride + water the equation is balanced because no.
What mass of water is produced?
What is the formula of hydrochloric acid?
.jpg)