This means we get a hydronium ion in this reaction. Hydrochloric acid is a colorless solution prepared by dissolving hydrogen chloride (hcl) in water. Following steps are followed to draw theh 2 so 4 lewis structure and they are explained.
Hydrochloric acid Molecular Geometry Molecular Weight
The eyes, skin, and hydrochloric acid are corrosive to the eyes, skin, and mucous membranes.
When you draw the structure remember that hydrogen (h) only needs two valence electrons to have a full outer shell.
Most stable lewis structure of h 2 so 4 is shown below. Science chemistry q&a library write a chemical equation using lewis structures for the reaction of hydrochloric acid with ammonia. Ci :) h+ [ci :) o none of the above. As a result, the chlorine follows the octet rule and has 8 electrons surrounding it on the one terminal of the
Select the correct answer below:
Hydrochloric acid is a strong, corrosive acid that can be used industrially to process steel used in the building and construction industry. 8 rows both hydrogen chloride and hydrochloric acid are corrosive. It is a colorless solution with a distinctive pungent smell. It is a strong corrosive acid with a chemical formula hcl.
Hydrochloric acid cannot be classified as a lewis acid since it cannot accept an electron pair.
Question 15 2 points identify the lewis structure of hydrochloric acid. It is classified as a strong acid. Cl :) h+[c] :] none of the above question : Hydrochloric acid is a monoprotic acid.
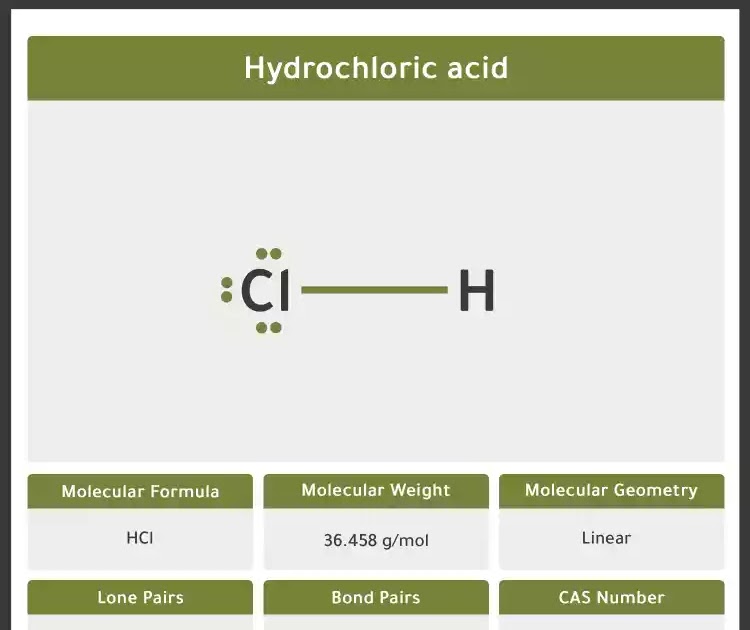
Diagram 1 shows the lewis dot structure of hydrogen chloride.
Hydrochloric acid lewis dot structure. C (group 4a) carbon (c): Structure, properties, spectra, suppliers and links for: The molar mass of hydrochloric acid is about 3605 g/mol.
6 rows drawing the lewis structure for hcl (hydrochloric acid) another straight forward lewis.
Hydrochloric acid [h + (aq) cl − (aq) or h 3 o + cl −], also known as muriatic acid, is an aqueous solution of hydrogen chloride (chemical formula: Draw an h for one hydrogen and a cl for one chlorine. Hydrochloric acid has many uses. Question 15 2 points identify the lewis structure of hydrochloric acid.
It means, it can release two hydrogen atoms to show acidic characteristics.
It is primarily used to show the. Drawing the dot structure for hydrochloric acid (hcl) and answer the questions below. Hydrochloric acid is a strong acid with the chemical formula hcl. Due to its inability to accept electron pairs, hydrochloric acid is often referred to as a classical acid rather than a.
Solved identify the lewis structure of hydrochloric acid.
The lewis structure for hcl (hydrochloric acid) is one of the easier dot structures to draw. Identify the lewis structure of hydrochloric acid. Watch the video of dr. Although hydrogen chloride and hydrochloric acid have the same chemical formula, one is a gas and the other is an aqueous solution.
The reason that the hydrogen and chlorine ions dissociate when in an aqueous solution is that hcl is polar covalent compound (see structure and properties for explanation on.
Hydrochloric acid has a molecular mass of 36.46 g/mol. Hydrogen (column number i) has one valence. It is a simple diatomic molecule. O (group 6a) oxygen (o):
Lewis structure of h 2 so 4.
Hydrochloric acid (hcl) is an arrhenius acid, this can be confirmed from the chemical equation given below which shows the reaction of hydrochloric acid (hcl) with water. However, this compound dissociates into its constituent ions, liberating h + ions (which are considered as lewis acids). It is very corrosive in its concentrated form. When hydrogen chloride is dissolved in water hcl is formed.
Determine the number of valence electrons for hydrogen and for chlorine by looking up the column numbers in the periodic chart for those elements.
Write a chemical equation using lewis structures for the reaction of hydrochloric acid with ammonia. Does hydrochloric acid qualify as a lewis acid? Steps of drawing lewis structure of h 2 so 4. With the help of one single bond, it already shares 8 electrons.
H c l + h 2 o → h 3 o + + c l −.
Select the correct answer below: Draw the elemental symbols for hydrogen and for chlorine. It is also known as hydrogen chloride or muriatic acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans.