Though the structure seems simple many underlying complexities to are going to be discussed in this article. Sih4 (silicon tetrahydride) vsepr geometry: Beh2 (beryllium dihydride) vsepr geometry:
Electron Dot Diagram For Hydrogen Chloride Diagram Media
H is a lewis acid when it forms an adduct with a lewis base.
When you draw the structure remember that hydrogen (h) only needs two valence electrons to have a full outer shell.
The lewis structure for hcl is a singly bonded h atom and cl schematron.org the cl atom there is one pair of dots on each unbonded side.how do you write the electron dot diagram of a hydrogen. Remember that hydrogen only needs two electrons to have a full outer shell. Drawing the lewis structure for hcl (hydrochloric acid) another straight forward lewis structure. Determine the number of valence electrons for hydrogen
Watch the video of dr.
Before going to the hybridization of hydrochloric acid, it is important to know the lewis dot structure of it. [3 points] calculate the amount of no+ produced in mmoles) that forms if the 0.95 g of sodium nitrite completely reacts with excess hydrochloric acid (assume a 1:1 stoichiometric ratio of sodium nitrite to nitrosonium.) Indicate the vsepr geometry about the central atom. Place the s atom at the center.
Number of electrons by o = 6 (3) number of electrons by o = 18 e.
Lewis structure of h 2 so 4. We draw lewis structures to predict. Drawing the lewis structure for hcl. Ocl2 (oxygen dichloride) vsepr geometry:
So that's what the lewis dot structure of this molecule would look like.
It is still used in the treatment of cellulose to make ethylcellulose for commercial products. Number of electrons by h = 1 e. The formal charge on the chlorine atom of hcl molecule= (v. What is the charge of h in hcl?
[2 points) draw the lewis dot structure of no.
Steps of drawing lewis structure of h 2 so 4. In resonance structures we mostly prefer 1st two structures because 3rd one have high charge so it is very unstable form then the other two. Hcn lewis dot structure consist of 3 elements as shown in the formula. Draw the elemental symbols for hydrogen and for chlorine.
Total in hno3 = 24 e.
Draw an h for one hydrogen and a cl for one chlorine. Number of electrons by n = 5 e. Hcl (hydrochloric acid) vsepr geometry: [3 points] draw the lewis dot structure of no*.
Identify this compound as either a nucleophile or an electrophile.
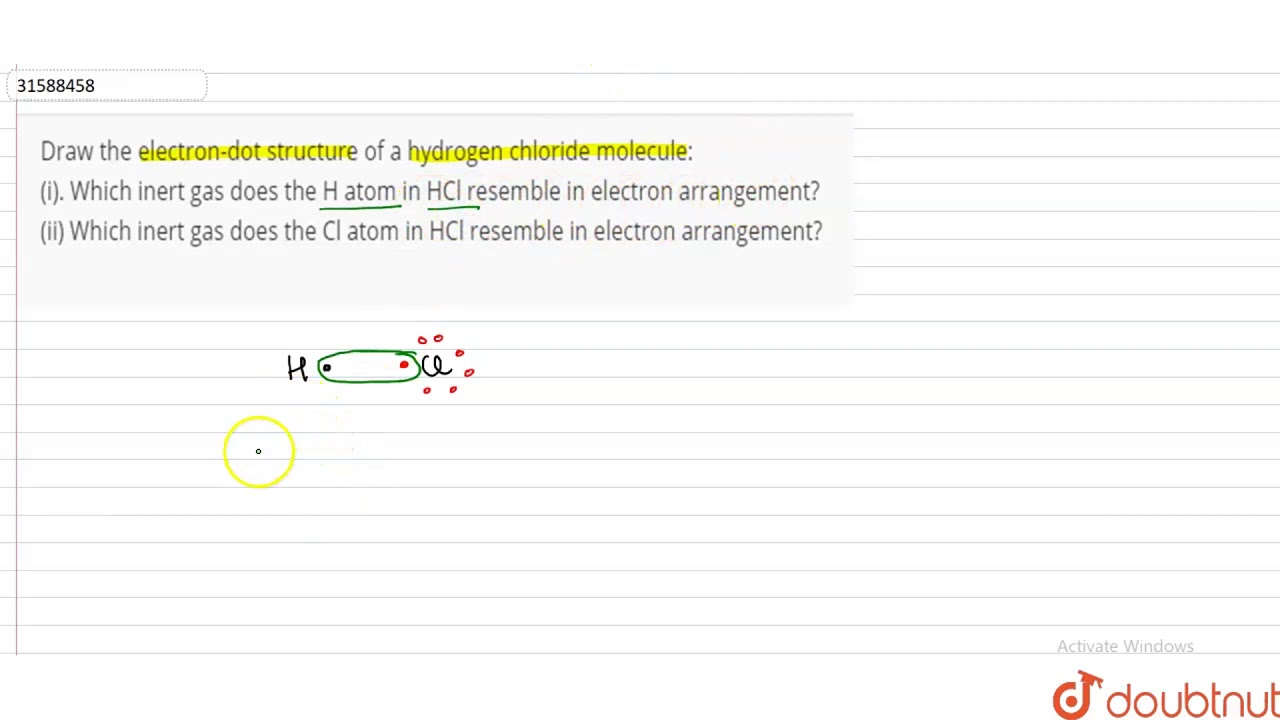
You have a total of 8 valence electrons available to fill the octets of chlorine and hydrogen in the hcl lewis structure. The h has a full outer shell of two electrons and the chlorine has a full outer shell of eight electrons. Lewis dot structure for hcl 4 of 6 the lewis structure for hcl hydrochloric acid is one of the easier dot structures to draw. What is the electron dot structure of hcl?
Using the lewis dot structure to predict bonding shape and type.
It is used in the production of chlorides, fertilizers, and dyes, in electroplating, and in the photographic, textile, and rubber industries. To calculate the formal charge on the central chlorine atom of the hcl molecule by using the following formula: Draw a lewis structure for the following. Chlorine, being a halogen, needs one electron to complete its octet.
Sodium nitrite and excess hydrochloric acid react to produce no*, also known as nitrosonium.
Let’s do a lewis dot structure drawing for hydrochloric acid, or hcl. Most stable lewis structure of h 2 so 4 is shown below. Colorpurplethus hcl acts as a lewis acid. Secl2 lewis structure selenium dichloride secl2 is a chemical formula for selenium dichloride.
Draw an h for one hydrogen and a cl for one chlorine.
Hydrochloric acid lewis dot structure draw the elemental symbols for hydrogen and for chlorine. Hydrochloric acid is corrosive to the eyes, skin, and mucous membranes. Hydrochloric acid lewis dot structure. If we wanted to show the lewis structure of hcl, we would draw the following:
It means, it can release two hydrogen atoms to show acidic characteristics.
The lewis structure of so2cl2 is constructed as follows. Drawing the dot structure for hydrochloric acid (hcl) and answer the questions below. Draw the lewis dot structures for the following. Hcn lewis dot structure is of great significance in terms of understanding the number of bond pairs, lone pairs, and type of bonds involved.
When you draw the structure remember that hydrogen (h) only needs.
In simple words, lewis dot structure is the distribution of electrons around the atoms which helps us to find out the number and types of bonds in the compound. Lewis dot structure for hcl (4 of 6) the lewis structure for hcl (hydrochloric acid) is one of the easier dot structures to draw. Hydrochloric acid has many uses. Determine the number of valence electrons for hydrogen and for chlorine by looking up the column numbers in the periodic chart for those elements.
Both hydrogen chloride and hydrochloric acid are corrosive.
Following steps are followed to draw theh 2 so 4 lewis structure and they are explained. The lewis structure for hcl (hydrochloric acid) is one of the easier dot structures to draw. All right, so hydrochloric acid is a really simple lewis dot structure.