Hydrochloric acid [h + (aq) cl − (aq) or h 3 o + cl − ], also known as muriatic acid, is an aqueous solution of hydrogen chloride ( ). Hydrochloric acid is a strong acid which used widely in various laboratory reagents, cleaning products, and the production of many industrial chemicals. By the alchemist jabir ibn hayyan (geber), by mixing common salt with vitriol (sulfuric acid).jabir discovered many important chemicals, and recorded his findings in over 20 books, which carried his chemical knowledge of hydrochloric acid and other basic chemicals for hundreds of years.
Chemical Structures of Acids Chemical structure, Organic
Hcl also known as marine acid, chloronium, spirit of salt.
Thus, we use hcl for both.
Most acids contain the element hydrogen, which serves as the donated proton when the acid dissociates in water. Hydrochloric acid is a component. Laguna design / getty images. The type of bond formed between the two ions depends on the two ions’ electronegativity.
6 rows hydrochloric acid hcl.
Hydrochloric acid is a clear, highly corrosive strong acid. The hydrogen is combined with the chlorine atom with a covalent bond and has a planar structure. Structural formula of hydrochloric acid. The molar mass being 36.46 g/mol, compound has a density of 1.18 g/cm3.
It is the solution of hydrogen chloride in water, and hcl is used synonymously for both the gaseous form and the aqueous solution.
Such a difference is also determined by the environment in. Hydrochloric acid is a colourless and odourless solution of hydrogen chloride and water; Similarly, the molecular weight of the same is 36.47 g/mol. This is the chemical structure of hydrochloric acid:
H c l + h 2 o → h 3 o + + c l −.
It is a highly corrosive, strong mineral acid with many industrial uses As an important chemical raw material, hydrochloric acid widely exists in various chemical production processes. Alkali, in the form of soda , has been used for centuries in the. This means we get a hydronium ion in this reaction.
Hydrochloric acid has many uses.
In hcl, the hydrogen is bonded to the chlorine atom with a covalent bond and has a planar structure. It is a colorless solution with a distinctive pungent smell. It's found in diluted form as muriatic acid. 8 rows both hydrogen chloride and hydrochloric acid are corrosive.
The molar mass or the molecular weight of hydrochloric acid is 36.5 g / mol.
It contains a hydrogen atom bonded to a chlorine atom. Hydrochloric acid (hcl) is an arrhenius acid, this can be confirmed from the chemical equation given below which shows the reaction of hydrochloric acid (hcl) with water. Hydrochloric acid was first discovered around 800 c.e. The chemical has many industrial and lab.
26 chemical structure of hydrochloric acid premium video footage.
Hydrochloric acid or muriatic acid is an aqueous solution of hydrogen chloride gas with the chemical formula hcl. This is an image gallery of the chemical structures of acids.these include the strong acids, such as hydrochloric and nitric acid, as well as important weak acids. Articles of hydrochloric acid are included as well. The molecular formula of hydrochloric acid is hcl.
The structure of hydrochloric acid or muriatic acid is represented as,
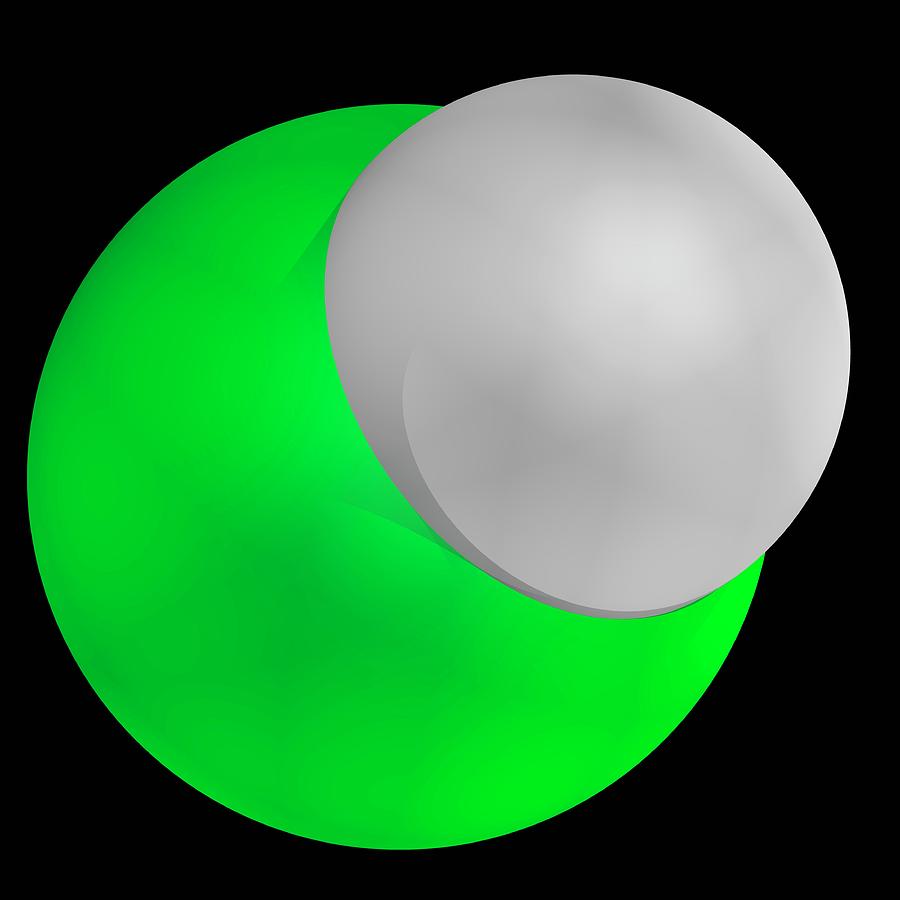
As the molecular formula suggests, hydrogen chloride is comprised of. Chlorine (green) and hydrogen (white). Its chemical formula is, h + cl ⇢ hcl. Other than that, we see that it is basically the solution of hydrogen chloride in water.
Hydrochloric acid has 2 elements named hydrogen and chlorine.
Hydrochloric acid is a simple diatomic molecule containing hydrogen and chlorine atoms. The structure of hydrochloric acid can be given as, hydrochloric acid has a linear shape. Amino acids are also listed. The chemical formula of hydrogen chloride is {eq}hcl {/eq}.
It was historically produced from rock salt.
After being used in industrial production, an.





/hydrogen-chloride-molecular-structure-on-white-475680141-5af19fd03037130036d25b94.jpg)