Start date oct 30, 2005; The hydrogen atom hamiltonian is by now familiar to you. The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom.
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts
Spectrum of hydrogen at the time of rutherford’s experiments, chemists analyzed chemical components using spectroscopy, and physicists tried to find what kind of order in complex spectral lines.
Oct 30, 2005 #1 pengwuino.
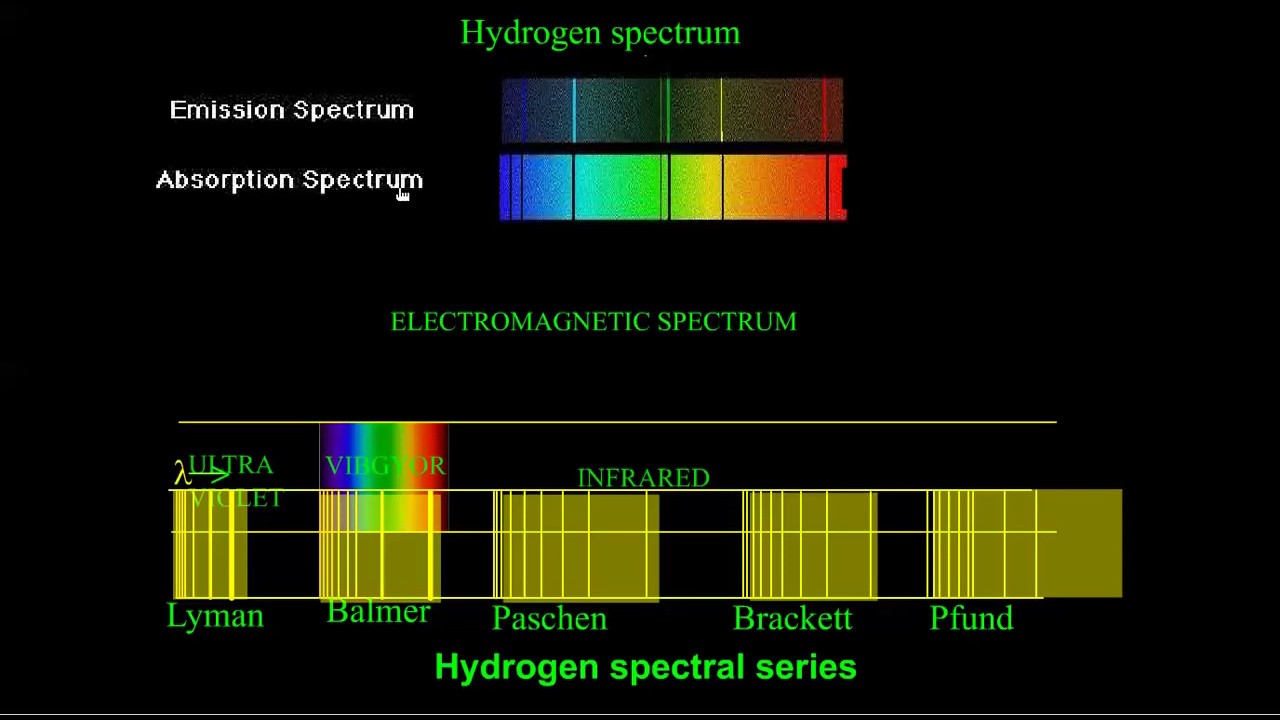
It was the birth of quantum mechanics! A hydrogen discharge tube is a slim tube containing hydrogen gas at low pressure with an electrode at each end. On the other hand, an absorption spectrum is constituted by the frequencies of light transmitted with dark bands. From the image above, it is evident that the atomic hydrogen emission spectrum is divided into a number of spectral lines with wavelengths given by the rydberg formula.
Measuring the lines of the hydrogen spectrum the visible portion of the hydrogen spectrum consists of four lines, as shown in the figure below.
When an atom absorbs a quantum of energy, it is said to be in an excited state relative to its normal (ground) state.when an excited atom returns to the ground state, it emits light.the frequencies of light emitted in such cases form the emission spectrum. However, only three of these lines are clearly visible using the spectroscope. Passing the light through a prism produces a line spectrum, indicating that this light is composed of photons of four visible wavelengths, as shown in figure 1. The spectral series are important in.
When white light passes through a gas and we analyse transmitted light using a spectrometer we find some dark lines in the spectrum.
Spectrum of the hydrogen atom. (2) where e is the charge on the electron and ε0 the permitivity of vacuum. The spectrum emitted by atomic hydrogen is shown. How many wavelengths of light can.
It also looks at how the spectrum can be used to find the ionisation energy of hydrogen.
The hydrogen atom hamiltonian is by now familiar to you. When a photon is emitted via a hydrogen atom, the electron undergoes a transition starting from a higher energy level to a lower, n = 3, n = 2, as an. Electrons in a hydrogen atom circle around a nucleus. For example, a hydrogen arc tube containing hydrogen, a light element, shows a highly ordered spectrum compared with other elements.
The classification of the series by the rydberg formula was important in the development of quantum mechanics.
Because of the electromagnetic force between the proton and electron, electrons go through numerous quantum states. The origin of spectral lines in the hydrogen atom (hydrogen spectrum) can be explained on the basis of bohr’s theory. Overview excited hydrogen atoms are. The spectrum shows a small molecular ion and a small peak resulting from loss of a hydrogen atom from the alcohol.
Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental value of 486.1 nm for the blue line in the visible spectrum of the hydrogen atom.
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the rydberg formula. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. Study of emission line spectra of a material can therefore serve as a type of “fingerprint” for identification of gas. The differences between energies of the excited states of the hydrogen atom determine the possible wavelengths, or alternately the frequencies, of photons emitted when excited electrons drop to lower energy states.
The gas gets dissociated and the hydrogen atom goes into an excited state and passes different radiations.
The balmer series, or balmer lines in atomic physics, is one of a set of six named series describing the spectral line emissions of the hydrogen atom. He hypothesizes that the angular momentum of an electron in orbit around a proton is quantized (i.e. Understand the experiment, apparatus, and procedures well. Under this simple assumption he managed to compute the energy of the electron around the atom:
When the hydrogen atom absorbs a photon, it results in causing the electron to experience a transition to the higher energy level, where n = 1, n = 2 as an example.
The visible spectrum of light from hydrogen displays four wavelengths, 410 nm, 434 nm, 486 nm, and 656. Neil bohr’s model helps in visualizing these quantum states as electrons orbit the nucleus in different directions. What is an emission spectrum? Hydrogen atom spectrum 29 5 spectrum of the hydrogen atom objective to calculate the rydberg constant from the spectrum of atomic hydrogen.
The observed spectral lines in the hydrogen emission spectrum are due to the atomic transitions between different energy levels.
The set of possible photon wavelengths is called the hydrogen atom spectrum. It can only be a discrete multiple of a certain number): These observed spectral lines are due to the electron making transitions between two energy levels in an atom. You will be performing many of the operations in the dark.
The hydrogen spectrum is formed when an electric discharge passes through gaseous hydrogen molecules.
H atom spectrum thread starter pengwuino; The hydrogen atom is said to be stable when the electron present in it revolves around the nucleus in the first orbit having the principal quantum number n = 1. Record the angle and color for each of the three lines in the spectrum. Your instructor will demonstrate how to use the spectroscope.
Atomic theory iv chemistry visionlearning from visionlearning.com.
Assume that a hydrogen atom's electron has been excited to the n = 9 level. The balmer series is calculated using the balmer formula, an empirical equation discovered by johann balmer in 1885. Spectrum of the hydrogen atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule.
If you put a high voltage across this (say, 5000 volts), the tube lights up with a bright pink glow.
Spectral series of hydrogen atom.