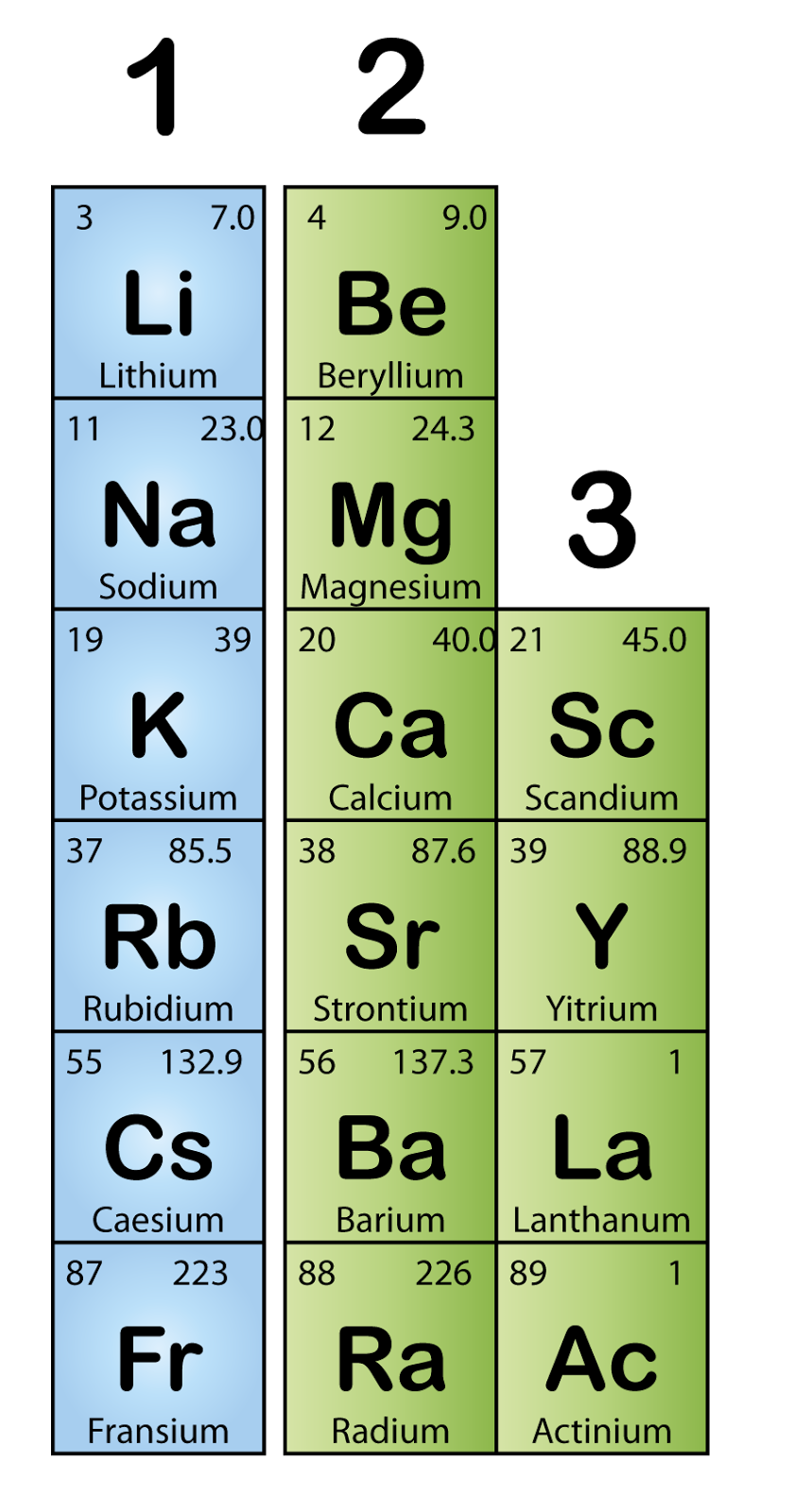
The first vertical column in the periodic table is referred to as group 1. The reactivity of this family increases as you move down the table. The table to the right shows the electron arrangement of all the group 1 metals.
Group One Metals Kamasutra Porn Videos
Group 1 elements are also known as alkali metals.
Group 1 comprise of neodymium, lithium, sodium, potassium,.
They have the least nuclear charge in their respective periods. Group 1 metals are all shiny, highly reactive, and very soft (we can easily cut them using a simple knife). Some of the important properties of carbonates are: Lithium, sodium, potassium, rubidium, cesium, francium.
It is usually measured on the pauling scale, on which the most electronegative element (fluorine) is given an electronegativity of 4.0 (table a2).
The group 1 elements are all soft, reactive metals with low melting points. As we move down the group, the atomic radius increases. They form alkaline solutions when they react with water. Atomic radius increases down a group, so the volume of the atoms also increases.
All the atoms of group 1 metal consist of 1 valence electron.
Beacon properties group has developed, managed, bought, and sold commercial real estate in the research triangle region of north carolina for over 30 years. They have a strong tendency to donate their valence electron in the last shell to form strong ionic bonds. All the metals react : Sodium chloride is used as a preservative for meat and to melt the ice on the roads (via freezing point depression).
Group 1 metals are very reactive metals.
This means that the alkali metals all have similar chemical properties. Metals are very reactive with chemical reactivity increasing down the group. All the atoms of group 1 metal consist of 1 valence electron. Alkali metals have low melting and boiling points as compared to heavy metals such as copper and iron.
The alkali metals share similar physical properties.
Group 1 metals are very reactive metals. When heated, the metal carbonate gets decomposed and liberates carbon dioxide (co2). The group 1 metals are known as the alkali metals. What are the properties of group 1 alkali metals?
Alkali metals are good conductors of heat and electricity.
Physical properties of group 1 metals the table below summarises the physical properties of the group 1 elements. Lithium, sodium, potassium, rubidium, cesium, francium. The table to the right shows the electron arrangement of all the group 1 metals. Furthermore, they have distinct flame colours, so we can easily distinguish them by exposing a sample to a bunsen burner.
Due to their low ionization energy, these metals have low melting points and are highly reactive.
Atoms of group 1 elements all have one electron in their outer shell. Learn vocabulary, terms, and more with flashcards, games, and other study tools. In an atom of ‘s’ block elements, the last electron enters ‘s’ orbital of the outermost orbit. :with oxygen to form oxides e.g.
Alternative names for group 1 are:
They all show the same chemical properties. Atomic and physical properties of the group 1 elements. They all show the same chemical properties. Alkali metals have low densities as compared to heavy metals such as iron and copper.
The carbonate ions have a planar structure due to which they are soft, soluble in hydrochloric acid, and have a significant anisotropy in many physical properties due to the planar form of the carbonate ion.
Silvery solids stored in oil. (a) alkali metals (1) (still commonly used) Group one elements share common characteristics. Start studying group 1 metals properties.
The alkali metals share similar characteristic chemical properties because they each have one.
They are all soft, silver metals. Chemical properties of the group 1 elements. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
The general electronic configuration of elements of group 1 is ns 1.
You will find separate sections below covering the trends in atomic radius, first ionisation energy, electronegativity, melting and boiling points, and density. The elements in group 1 of the periodic table are called the alkali metals. They react with water to produce an alkaline metal hydroxide solution and. Element melting point °c boiling point °c density kg/m3 atomic radius nanometer (nm) li 180 1342 535 0.15 na 98 883 968 0.19 k 64 759 856 0.23 rb 39 688 1532 0.25 cs 29 671 1879 0.26 alkali metals have lower melting and boiling points all group 1 elements.
They include lithium (li), sodium (na) and potassium (k).
Are soft (they can be cut with a knife) have relatively low melting. Alkali metals are soft solids and can be easily cut. The alkali metals all the alkali metals react vigorously with halogens to produce salts, the most industrially important of which are nacl and kcl. Trends in properties of group 1 elements (alkali metals) chemistry tutorial key concepts.