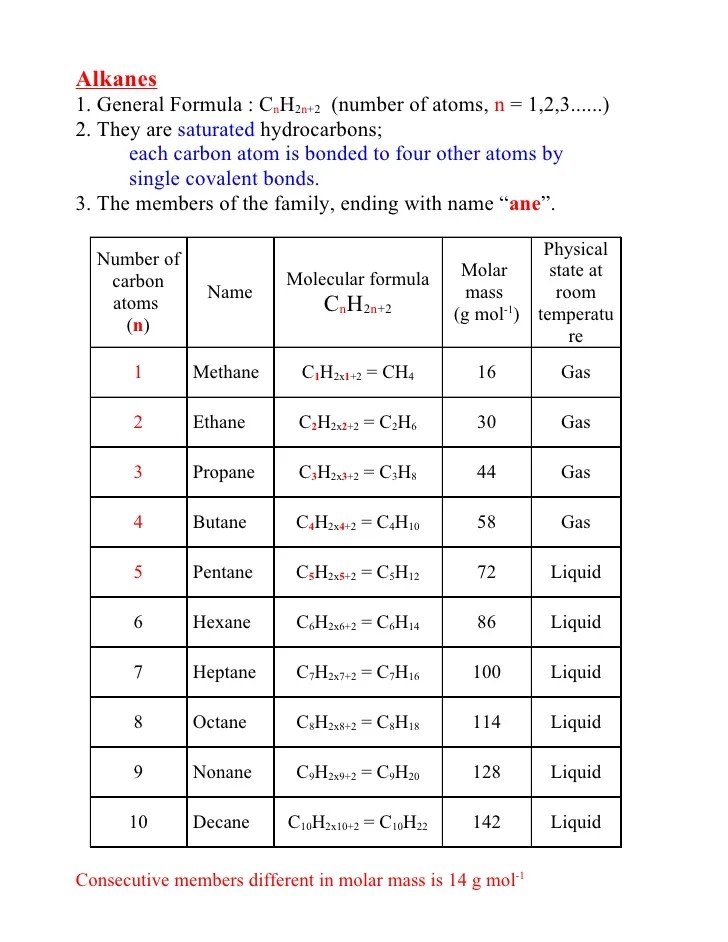
Alkanes are the simplest hydrocarbons known to us. C x n h 2 n + 2. The general formula for the alkenes is [ {c_n} {h_ {2n}}] , where n is the number of carbon atoms in the molecule.
Chemistry Notes Edexcel Unit 1 2 and 3 Notes Chemistry
Alkanes belong to the family of saturated hydrocarbons
As with other organic compounds, the carbon atoms in alkanes may form straight chains, branched.
The alkanes are also called as paraffins. For example, an alkane with 2(n) carbon atoms, will have 6 (2n+2) hydrogen atoms. By reason of their formula alkanes are said to have no degrees of unsaturation. When two or more branched chains are present, give each branched.
C x n h 2 n + 2.
The most important is the first member of the series, the etino or acetylene of the formula hc≡ch. Iupac nomenclature of alkanes, alkynes, and alkenes are explained below: A brief structure shows only the bonds as in the compound on the extreme left. Alkenes are often used as a synonym of olefin.
The generic formula for alkanes is cnh2n+2, where n is the number identified by the prefix.
First member is ethyne c 2 h 2. This method of naming is known as iupac naming or iupac nomenclature. A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2: Please log in or register to add a comment.
Methane gas is the first member of the homologous series of alkanes.
\[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two. The general formula of alkenes is c n h 2n. There is no alkyne with one carbon atom. Khan academy is a nonprofit organization.
C n h 2n alkyne:
This gives the parent name for the alkane 2. Number the parent chain beginning with the end of the chain nearer the branched chain 3. C n h 2n+2 alkene: Alkenes have at least one double bond and alkynes have at least one triple bond.
They have a general formula of c n h 2n+2.
The general formula for alkynes is cnh2n−2 c n h 2 n − 2. Alkanes have the general formula of c n h 2n + 2 where n is the number of carbon atoms. Alkanes have the general formula c n h 2n+2. The longest continuous chain should include both the carbon atoms of the triple bond.
Alkynes are the most unsaturated out of alkanes, alkenes and alkynes.
General formula for alkenes is cnh2n. Alkynes aliphatic hydrocarbons having general formula c. The straight chain alkanes share the same general formula: In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms.
What is the general formula of alkynes?
The name olefin is derived from the greek word olefin gas, which means oil forming. For cyclic alkanes, carbon must still have four bonds, hence each carbon is attached to two others and two hydrogens are groups may be attached to each carbon (structure on the right). Use the number obtained by application of rule 2 to designate the position of the branched chain 4. Pentacosane c25h52 is an example of a very long linear straight chain.
Please log in or register to add a comment.
The general formula for rings is c n h 2n. Iupac nomenclature of alkanes, alkynes, and alkenes. The most common alkyne is ethyne, better known as acetylene. What is the general formula of alkynes?
The general formula for alkanes is cnh2n+2, where n stands for number of carbon atoms and 2n+2 for number of hydrogen atoms in the molecule.
Alkanes have the general formula c n h 2n+2 and can be subdivided into the following three groups: Let's look at the members of the alkane, alkene, and alkyne family and see what is their general molecular formula. Where the formula is cnh 2n or cnh 2nom, each 2. Alkenes have the general formula c n h 2n.
This gives them a general formula :





![Alkane Formula [with free study guide]](https://i2.wp.com/www.aceorganicchem.com/blog/wp-content/uploads/2017/08/Alkanes.png)