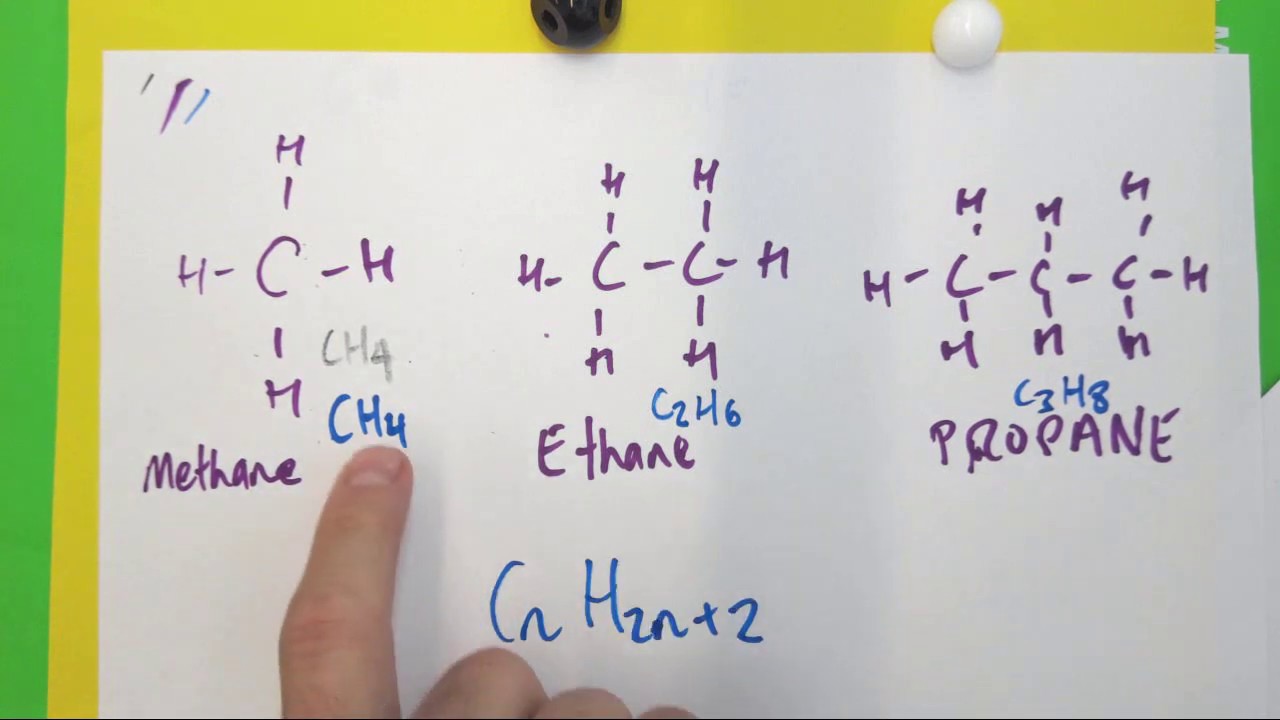
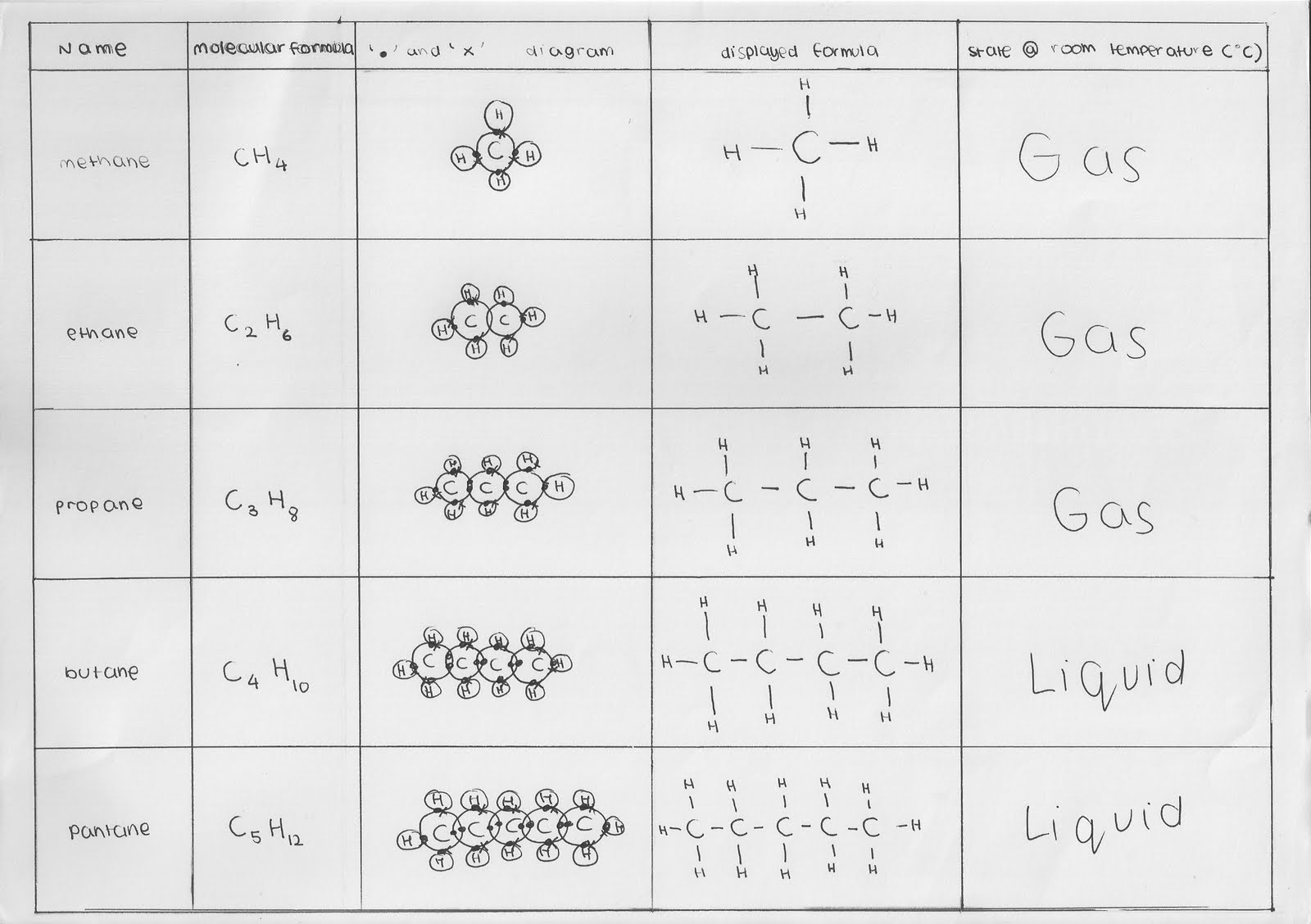
The general formula for alkane is c n h 2 n + 2 , where n is any integer equal to or greater than 1.the simplest alkane is methane having one carbon atom and molecular formula c h 4 , 2. If we take the general formula for an alkane, , then n is clearly 3. We offer quizzes, questions, instructional videos.
(a) Give the general formula of an (i) alkane,(ii
With respect to this formula, it is often useful to calculate the degree of unsaturation possessed by any given organic formula.
The general formula is cnh2n.
An alkane has general formula c_{n}h_{2n+2}, and try it out for methane, ethane,. By reason of their formula alkanes are said to have no degrees of unsaturation. Write general formula of alkanes and four general methods for preparation of alkanes. Alkanes have a general formula:
The general formula of alkanes is :
According to the formula, alkanes have hydrogen atoms. What is the general formula of alkynes? Each edge of the chemical structure corresponds to. A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2:
All alkenes have at least one double bond.
Substituting n for 3 gives the following: Alkynes aliphatic hydrocarbons having general formula c. By substituted is meant replacement of a hydrogen by some other atom or group of atoms, or the insertion of a different type of chemical bond in the molecule. Cnh 2n+2 is the generic alkane.
Cycloalkanes have one or more rings of carbon atoms.
The straight chain alkanes share the same general formula: In an alkane is double the number of carbon atoms, plus two. Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes. (i) the number of alkane family.
Alkenes and cycloalkanes have the same general formula, cnh2n.
The general formula for an alkane is c n h 2n + 2. The general formula for an alkane is cnh2n+2. Once you calculate this, for a specified formula you can predict how many unsaturated bonds, c = o, c = c, c = n, how many ring. Alkanes are acyclic aliphatic hydrocarbons having the general molecular formula cnh2n+2 [12].
C n h 2n alkyne:
Because they use only single bonds, alkanes contain the maximum number of hydrogen atoms relative to the number of carbon atoms, and are therefore said to be saturated. 4 rows the alkanes comprise a series of compounds that are composed of carbon and hydrogen atoms with. 10 rows the general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number. What does n stand for in cnh2n?
Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes.
Where the formula is cnh 2n or cnh 2nom, each 2 hydrogens less than 2n +2 represents a degree of unsaturation. Alkenes are the unsaturated hydrocarbons that. Hydrocarbons are molecules made from. Let's look at the members of the alkane, alkene, and alkyne family and see what is their general molecular formula.
Only carbon, hydrogen and oxygen.
C n h 2n+2 alkene: \[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms. The general formula of alkenes are c n h 2n in comparison to alkanes with general formula c n h 2n+2. What is the cyclic analogs of alkanes?
The simplest examples of this class consist of a single, unsubstituted carbon ring, and these form a homologous series similar to the unbranched alkanes.
Well, let us go thru it exhaustively….