For a first order reaction: T is the time elapsed since the reaction began Rate = k[a] integrated rate law (concentration vs.
Chemical Dynamics Lecture 2 Chemical (Part 2)
Increasing the concentration of the reacting species will increase the rate of the reaction.
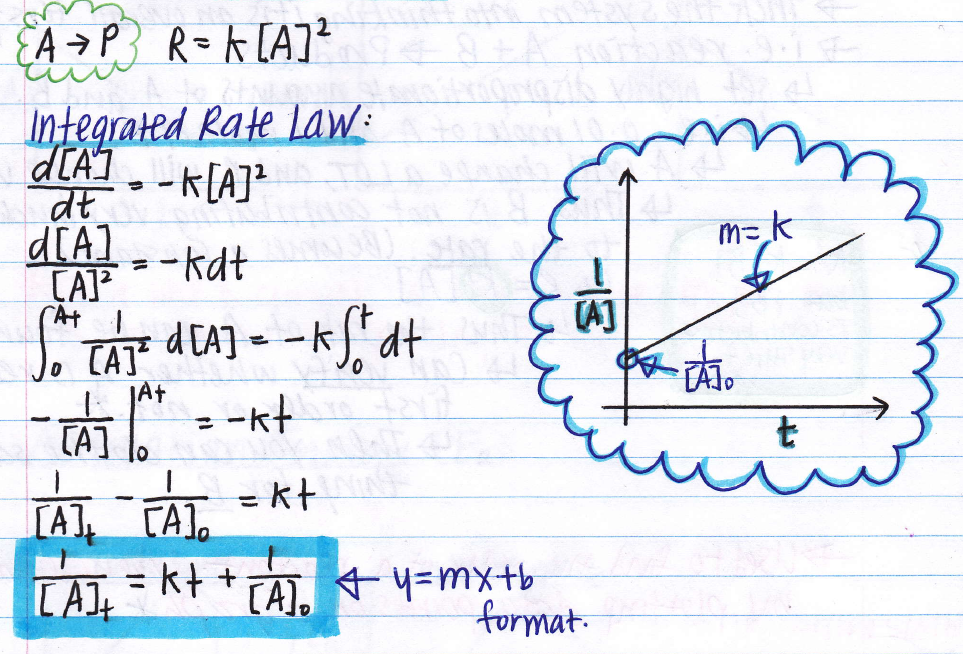
Integration of this ordinary differential equation is elementary, giving:
80% decomposed means new concentration is 0.2 m. If a set of rate data are plotted in this fashion but do not result in a straight line, the reaction is not first order in a. Table 17.1 integrated rate law summary; Reaction order integrated rate law characteristic kinetic plot slope of kinetic plot units of rate constant;
A plot of ln[a] vs.
Concentration and rate values, because the plot tends to average out all the experimental errors. Where k is the rate constant plus the natural. • the rate of a first order reaction is proportional to the concentration of the reactant. Therefore, the rate law for this reaction is,
How long (in s) will it take for 80.0% of the reactant to decompose?
Concentration at any moment of time can be given as, [a] = [ a] 0 e − k t. Mol l −1 s −1: A full integration of the equation can be found here. L mol −1 s −1
C 4 h 8 2c 2 h 4.
Ln [a] t = −kt + ln [a] 0: The first order integrated rate equation first order rate law chem 3310 2 first order (m+n=1) this is the first order differential rate law. Consider the reaction r → p again. Ln[a] = ( − k)(t) + ln[a]0 y = mx + b.
2a products or a + b products (when [a] = [b]) , rate = k[a] 2
The first order rate integral[1] equation calculates the rate at which the reactants turn in to products. In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that is the rate of the reaction is proportional to the first power of the concentration of the reactant. [a] = [a]o(½) h t½ = 0.693/k Jay courses 455 view detail preview site
Assume initial 1 l solution and 1 m concentration.
Thus, we can determine the concentration and rate of reaction at any moment with the help of integrated rate equation for zero and first order reaction. Rate = k[nh 3] 0 = k.