Rate law for this reaction: (use the integrated rate laws and graphing to get orders). The slope of the straight line signifies the value of rate constant, k.
Derive Integrated Rate Equation For First Order Reaction
A) order of each reactant b) rate law expression c) overall order of the reaction d) value of the rate constant with proper units 1981 experiment initial conc.
The expressions for the change in concentration with time are differential equations which, in some cases, can be integrated to give a different, but related, form of the rate equation.
Up to 24% cash back rate law equation worksheet 1 answers part 1: Rate law equation practice 1. The rate constant, by definition, is independent of concentrations, initial or otherwise, so ( a) must be true. The rate of this rxn depends only on no 2:
Although these sound complicated, they are actually quite simple.
They are used to describe what is happening at the molecular level during a reaction. These rate laws help us determine the overall mechanism of. Differential rate law and integrated rate law are two forms of rate laws. [a] = [a]o(½) h t½ = 0.693/k
Differential and integrated rate laws practice problems.
Rate law and reaction order. Although the differential law relates the rate and concentrations of reagents, a second form of fee called integrated law relates concentrations of reagents and time. A plot of ln[a] vs. First, we need to discuss factors that affect the rate of a chemical reaction:
For a reaction where the rate equation is r = k[nh 4 + (aq) ][no 2 (aq) ], a) calculate k at temperature t 1 , if the rate, r, is 2.40x10 7 mol/(l⋅s) when [nh 4 +
Practice for the student 1. The rate of a reaction can be expressed by any one of the reactants. Rate = k[a] integrated rate law (concentration vs. 5 given a rate law, how much will rate change with change in concentration 20.
The integrated rate law can be rearranged to a standard linear equation format:
Kinetics practice problems and solutions determining rate law from time and concentration data. The key difference between differential rate law and integrated rate law is that differential rate law gives the rate of a chemical reaction as a function of the change in concentration of one or more reactants during a particular time period whereas integrated rate law gives the rate of a. What is the integrated rate law for a zero order reaction? The rate law for this reaction is second order in a and second order in b.
The formulas below are the integrated rate laws.
We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order and rate constant of a reaction. The expressions for the change in concentration with time are differential equations which, in some cases, can be integrated to give a different, but related, form of the rate equation. No 2 + co no + co 2. Order with respect to no 2:
Differential vs integrated rate laws differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time;
What is the differential rate law for a simple zero order reaction? Differential rate law practice for each data set below determine the: S after the start of the reaction. This is the currently selected item.
Differential rate law (rate vs.
Up to 10% cash back example question #1 : The differential and integrated rate laws in chemistry (and physics, biology, etc.) in general, for all reactions: The rate law is second order overall. This activity will focus primarily on the last two types, differential rate and integrated rate.
View intergrated rate law problems.pdf from chem 113 at queens university.
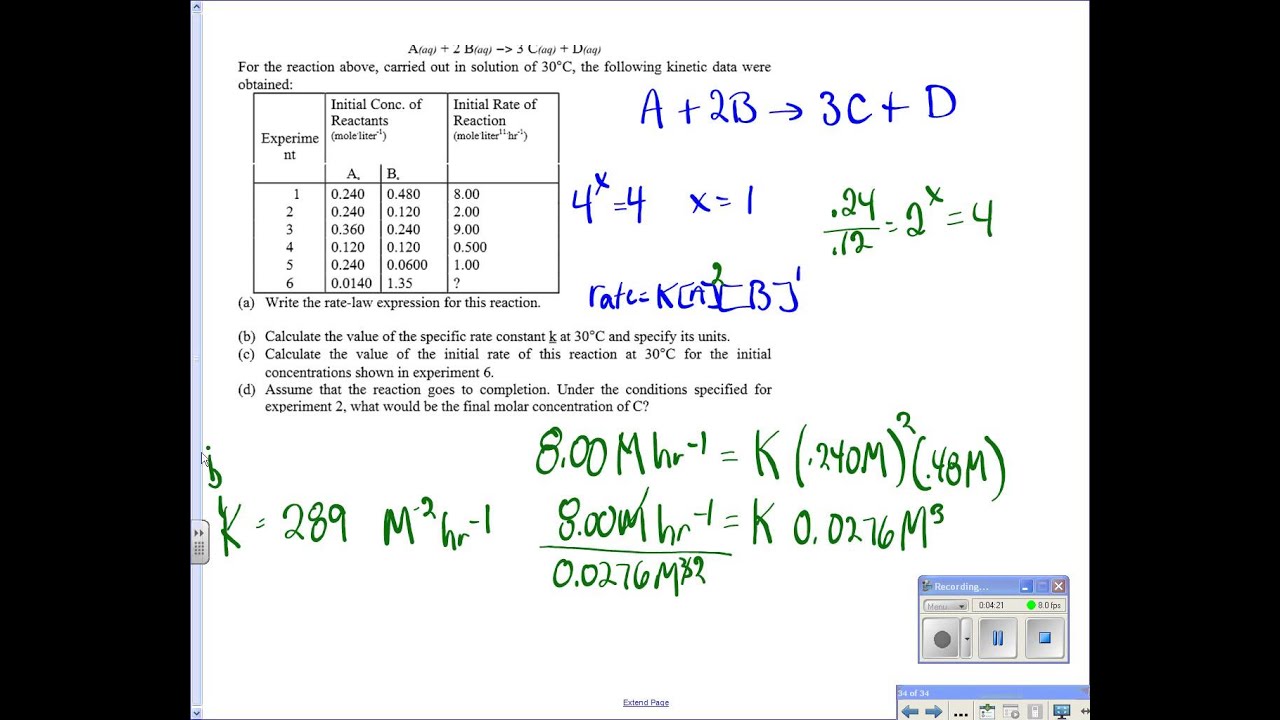
The rate law for this reaction is first order in a and first order in b. Of reactants (mole.liter−1) initial rate of reaction (mole.liter−1.hr−1) a o b o 1 0.240 0.480 8.00 The above equation is known as integrated rate equation for zero order reactions. Integrated rate laws can be used to determine the amount of reagent or product present after a period of time or to estimate the necessary time for a.
If the initial concentration of the reactant is 0.30 m, how long does it take for the concentration to decrease to 0.15 m?
We will not be doing the integration in this class, but we will be looking at the solutions to those integrations. What are the differential and integrated rate laws for a simple third; The following data were collected. Unless you have to answer this.
Consider the reaction a + 2b c + d.
How would you plot data to identify it as zero order? Problem that deal with rate laws in both the differential and integrated forms skills utilized: Integrated rate law and half life sample problems answers. Aa → bb + cc rate = − 1 𝑎𝑎 𝑑𝑑[𝐴𝐴] 𝑑𝑑𝑑𝑑 = 1 𝑏𝑏 𝑑𝑑[𝐵𝐵] 𝑑𝑑𝑑𝑑 = 1 𝑐𝑐 𝑑𝑑[𝐶𝐶] 𝑑𝑑𝑑𝑑.
Up to 24% cash back in order to create equations that can be used to calculate this information, the rate laws must be integrated over time.
Up to 24% cash back rate, instantaneous rate, differential rate, and integrated rate. Determining a rate law using initial rates data. The reaction chcl 3(g) + cl 2(g) → ccl 4(g) + hcl(g) has the following rate law: