An alkene represents an unsaturated hydrocarbon with double bonds, while an alkane is a saturated hydrocarbon with only single bonds. Check answer and solution for ab Capability to convert alkenes right into alkane is a vital chemical response that implies we might be able to take simple particles and update them into important asset chemicals, like hydrogenated oils and petrochemicals which can be utilized as energy resource.
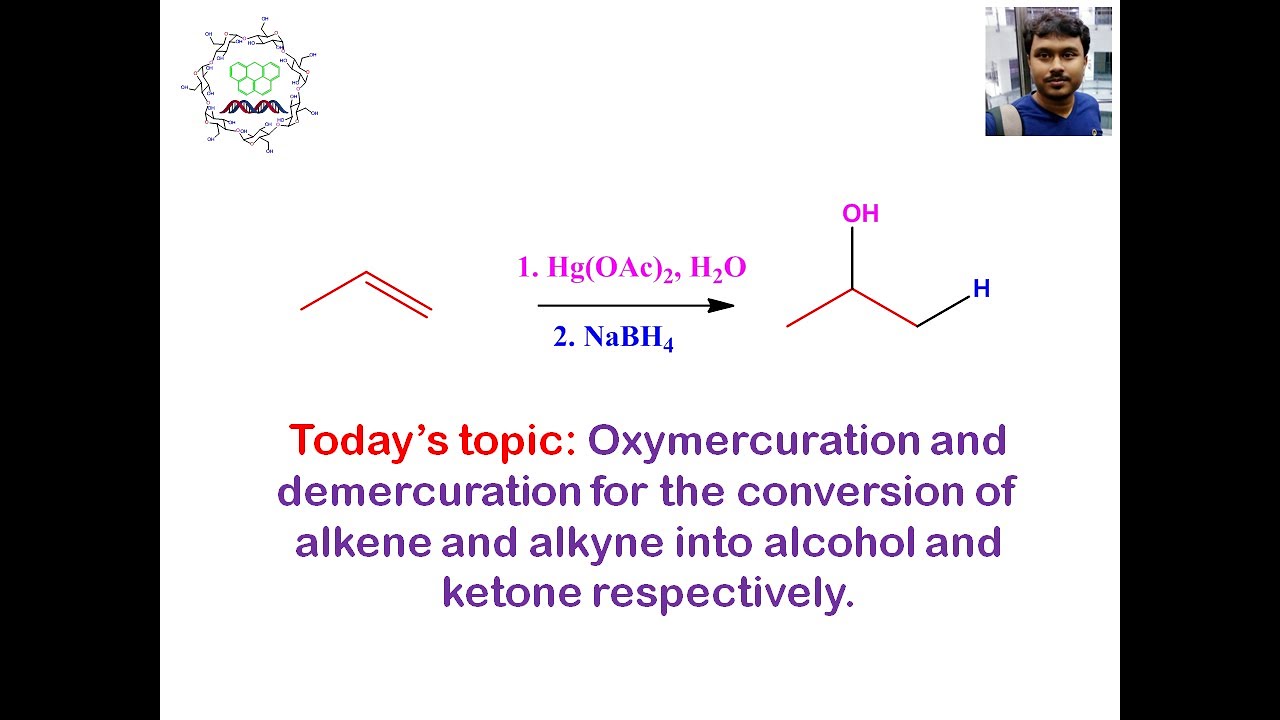
Oxymercuration and Demercuration conversion of alkene and
Advanced alkenes and alkynes topics.
An alkene represents an unsaturated hydrocarbon with double bonds, while an alkane is a saturated hydrocarbon with only single bonds.
2 topics | 2 quizzes. Halogenation goes via free radical mechanism and thus lot of unwanted products + other isomers can be formed. The alcohols may be considered as derivatives of alkanes. How do alkanes form alcohol?
Convert the alkane to halogen halide;
In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy). There are probably hundreds of possibilities at varying conditions to convert alkanes to alkenes. Then dissolve in water to get the alcohol. Alcohols can be prepared by the hydration of alkenes.
To convert an alkane to an alkene, requires that you remove hydrogen from the alkane molecule at extremely high temperatures.
This question is therefore very broad and you should probably try to narrow it down to add context and add your own research on possible reactions. Because they are isomeric with cycloalkanes or bicycloalkanes, their names must clearly convey. To make them react further we need to introduce some functional group and the best way is to add halogen. Contacting a hydrocarbon feedstock comprising lower alkanes and alkenes with a fluidized bed of acidic, shape selective metallosiliate catalyst in a first conversion zone under high temperature alkane conversion.
Treat it with magnesium metal and dry ether to form grignerd reagent.
How to convert a trans alkene into a cis alkene? This patent describes a process for the conversion of lower alkane and alkene hydrocarbons to high octane gasoline. If only one r group is an alkyl group and the other r is a hydrogen, that side of the double bond will become an aldehyde fragment. To convert an alkene to an alkane, you must break the double bond by adding hydrogen to an alkene in the presence of a nickel catalyst, at a temperature of around 302 degrees fahrenheit or 150 degrees celsius, a process known as hydrogenation.
Examples of mo’s in typical conjugated systems.
Conversion of alkene to alcohol by hydration: This invention provides for a process to convert branched or linear alkanes to branched or linear alpha olefins (ao) of the same carbon number. To convert an alkene to an alkane, you must break the double bond by adding hydrogen to an alkene in the presence of a nickel catalyst, at a temperature of around 302 degrees fahrenheit or 150 degrees celsius, a process known as hydrogenation. The synthesis of by converting alkene to alcohol is a commonly used method.
The method to convert alkene into alcohol is described below:
To convert an alkane to an alkene, requires that you remove hydrogen from the alkane molecule at extremely high temperatures. Alkane to alkene to alkyne alkanes are highly unreactive. One important alkene addition reaction is hydrogenation., where the alkene undergoes reduction to an alkane. Otherwise its score will drop, or it gets closed (or both) and you won't get an answer.
The first step is usually the.
An alkene represents an unsaturated hydrocarbon with double bonds, while an alkane is a saturated hydrocarbon with only single bonds. To convert an alkane to an alkene, requires that you remove hydrogen from the alkane molecule at extremely high temperatures. How do you convert alkenes to aldehydes? How do you reduce an alkane to an alkyne?
This process is known as dehydrogenation.
This process is known as dehydrogenation. Direct reduction of alcohols to alkanes is generally difficult. The ozonolysis of an alkene. If both r groups on one side of the double bond are alkyl groups, that side of the double bond will become a ketone fragment;