C 5 h 5 n & c 5 h 5 h + ammonia & conjugate acid: Calculate the ph of the resulting buffer solution. In nature, there are many systems that use buffering for ph.
pH of Buffer Solution (Example) YouTube
The k b of nh 3 is 1.77 x 10¯ 5.
Therefore, to make a buffer solution of a particular ph, we used a weak acid whose pka is close to the desired ph value.
Its ph changes very little when a small amount of strong acid or base is added to it. These are further of two types, acidic buffer: Calculate the ph of an unbuffered 0.010m acetic acid solution. Similarly, for alkali buffering, ph = pkb + log[salt]/[base], where pkb = dissociation constant of the.
A buffer solution is composed of a weak acid, and its conjugate base in appreciable concentrations.and so five examples are.
First, write the equation for the ionization of acetic acid and the k a expression. Phosphate buffer system (also occurs in the cells of the body) buffer of. Calculate the volume of 0.2m solution of acetic acid that needs to be added to 100 ml of 0.2m solution of sodium acetate to obtain a buffer solution of. Acid buffers are liquids with a ph of below 7, containing a weak acid and one of.
Mv = grams / molar mass (m) (0.250 l) = 7.45 g / 53.4916 g/mol
Assuming the change in volume when the sodium acetate is not significant, estimate the ph of the acetic acid/sodium acetate buffer solution. A buffer solution was made by dissolving 10.0 grams of sodium acetate in 200.0 ml of 1.00 m acetic acid. Weak acid and a salt of acids conjugate base in sufficient amounts are required to maintain the ability of buffer. It is the solution of a mixture of a weak base and a salt of this weak base with a strong.
1) we need the molarity of the ammonium chloride:
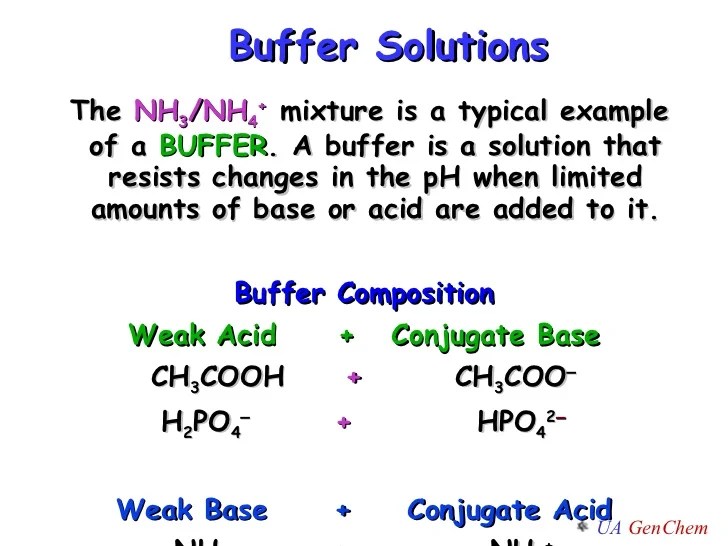
These are called acid buffers, (eg): An example of a buffer that consists of a weak base and its salt is a solution of ammonia (nh3(aq)) and ammonium chloride (nh4cl(aq)). What is buffer solution write 1 1 example for acidic and basic buffer? A buffer works by having both the weak acid/conjugate base or weak base/conjugate acid in a solution between the ratio of 1 to 10 and 10 to 1, with the most effective buffer being at a 1 to 1 ratio.
A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt.
What are buffer solutions used for? Alkaline buffers, on the other hand, have a ph above 7 and contain a weak base and one of its salts. An example of an alkaline buffer solution is a mixture of ammonium hydroxide and ammonium chloride (ph = 9.25). Ammonium acetate (ch3 coonh4, acts as a buffer.
Thus, examples of buffer solutions are h f and k f, acetic acid and sodium acetate, ammonia and ammonium chloride, etc.
What are buffer solutions examples? Buffer solutions are used as a means of keeping ph at a nearly constant value in a wide variety of chemical applications. In solution, the salt is completely ionized and the weak acid is partly ionized. Two common types of bufffer solutions are :
For example, a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a ph of about 9.25.
A weak base mid its salt with a strong acid. For example, a mixture of acetic acid and sodium acetate acts as a buffer solution with a ph of about 4.75. A common example would be a mixture of ethanoic acid and sodium ethanoate in solution. There are various other examples of buffer solutions, including:
(a) the buffered solution on the left and the unbuffered solution on the right have the.
Buffer solutions are used in a wide variety of chemical applications as a. An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride (nh 3 (aq) + nh 4 cl(aq)). (iii) bicarbonate/carbon dioxide (carbonic acid) (iv) dihydrogen phosphate/biphosphate. It is the solution of a mixture of a weak acid and a salt of this weak acid with a strong base.
A conjugate salt differs from its original acid or base by one h + ion or one o h − ion.
The balanced equation for this reaction is: Acetic acid & conjugate base: Nh 3 & nh 4 + methylamine & conjugate acid: For example, blood in the human body is a buffer solution.
Buffer solutions are used as a means of keeping ph at a nearly constant value in a wide variety of chemical applications.
A solution of acetic acid (ch3cooh and sodium acetate ch3coona) is an example of a buffer that consists of a weak acid and its salt. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. A weak acid together with a salt of the same acid with a strong base. In this case, if the solution contained equal molar concentrations of both the acid and the salt, it would have a ph of 4.76.