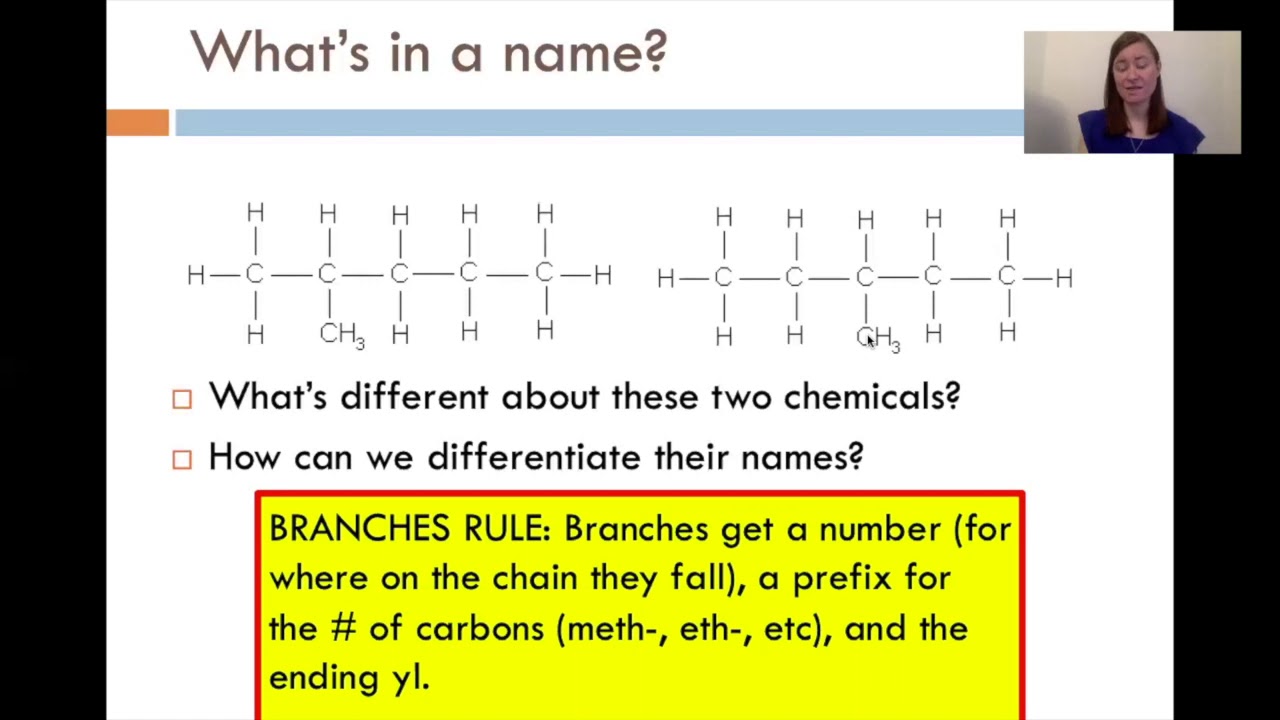
An example of an alkane isomer is a linear and a branched alkane sharing the same molecular formula. For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each. Choose the longest, most substituted carbon chain.
Nomenclature of Alkanes — Organic Chemistry Tutor
Cyclic alkane examples name these cyclic alkanes.
Inflammable and irritating vapor is produced (national center for biotechnology information, pubchem compound database, 2017).
The name of a branched alkane must also describe the positions of the parent chain which are connected to the. What are branched chain alkanes? The name given to the parent chain of a branched alkane is the same as the name of the corresponding linear alkane. What are the rules for naming branched alkanes?
It is a gas that occurs.
An alkane can be appended onto an existing chain to create a branched molecule. For example, in the alkane shown here, the longest chain is five c atoms long, so it is a pentane: Branches sprout from one or more atoms of the main skeleton (or from monomers along the chain of a polymer). It is an aqueous liquid with a gasoline odor that floats on water and produces an irritating vapor.
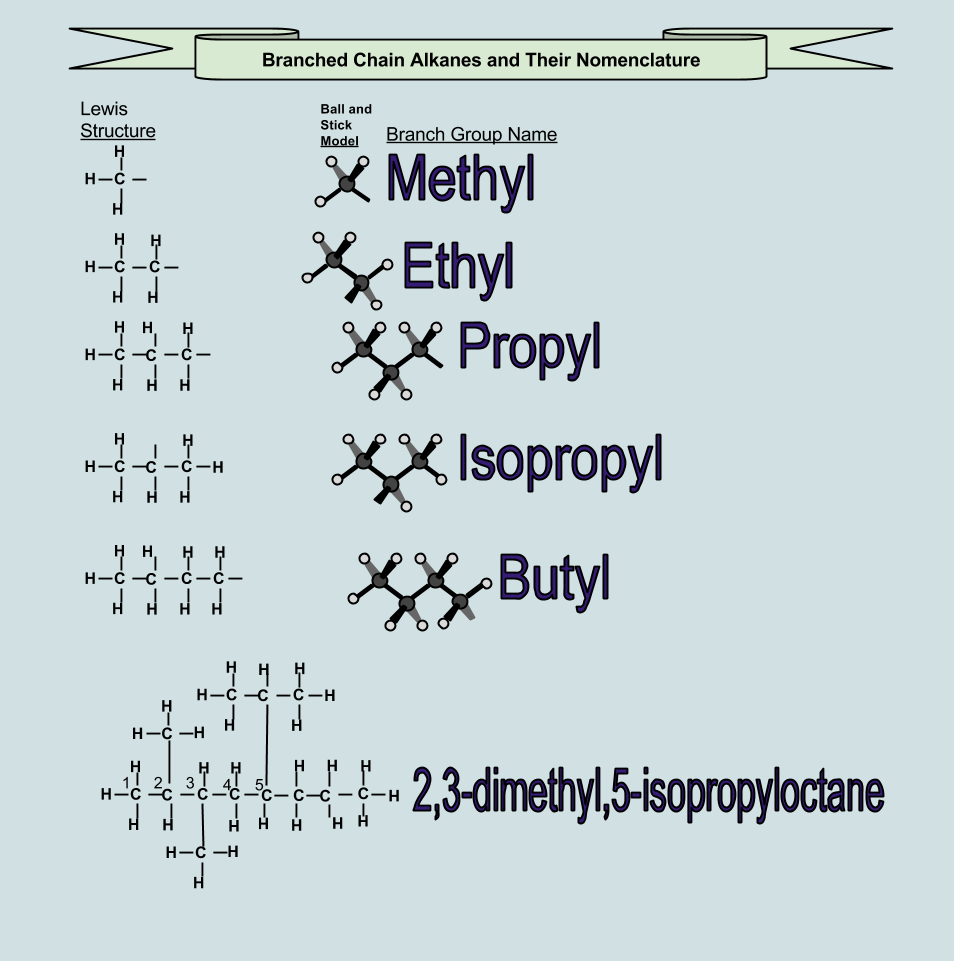
Methyl indicates there is one carbon atom in the branch.
Once again, it is very important to learn the names of the linear alkanes. What is a branched alkyl? Is a branched chain alkane of the molecular formula c 6 h1 4. In alkanes, hydrocarbons are the simplest organic compounds that are made by using only carbon and hydrogen atoms.
It is the simplest alkane and is made up of one carbon and four hydrogen atoms.
This branched piece of the molecule is called a substituent. The longest chain is highlighted in red and consists of eight carbons. Name the alkane shown below. If equal number of carbons, go with the cyclic portion as parent name.
Wrapping up practice naming acyclic and cyclic alkanes using iupac rules.
So, for example, a six carbon parent chain is named hexane. Thus open chain alliphatic alkanes are of two types 1) straight chains and 2)branched chains. Branched alkanes are alkanes where carbon chain is split off making one or more branch. These side chains are named as branches.
They each contain one carbon atom, so they are both methyl groups.
The name now becomes “methyloctane”. Cyclic alkanes are alkanes where two carbons in any carbon chain are linked together to form a chain. The general formula of cyclic alkanes are c n h 2n, n>3. Example of a branched alkane.
Therefore, these compounds are not linear hydrocarbons.
Some of the main uses of alkanes are: They contain branches with groups such as methyl, ethyl, coming off the main branch of the molecule. Hydrocarbons are also regarded as the primary organic substance. These items are extensively spread in nature and are used for various purposes.
Vapors from this substance used to be used as anesthetics, but were later banned because they were found to damage.
An example of such is pentane (linear), isopentane. Spiro [2.2]pentane is an alkane with two rings, so it is a cyclic alkane, with the general formula of c n h. Example of a branched alkane. Greater number of carbons takes priority.
Molecular skeleton is bonded to (at most) two other carbons.
Ethyl indicates there are two carbon atoms in the branch. Which molecule is a branched alkane? The rule is a comma between numbers, and a dash between numbers and. Some examples of such items are coal, petroleum and natural gas.
They contain branches with groups such as methyl, ethyl, coming off the main branch of the molecule.
The longest continuous chain is c5. Branched chain alkanes are hydrocarbon compounds containing side groups attached to a continuous carbon chain. At this point, the parent name is “octane”.