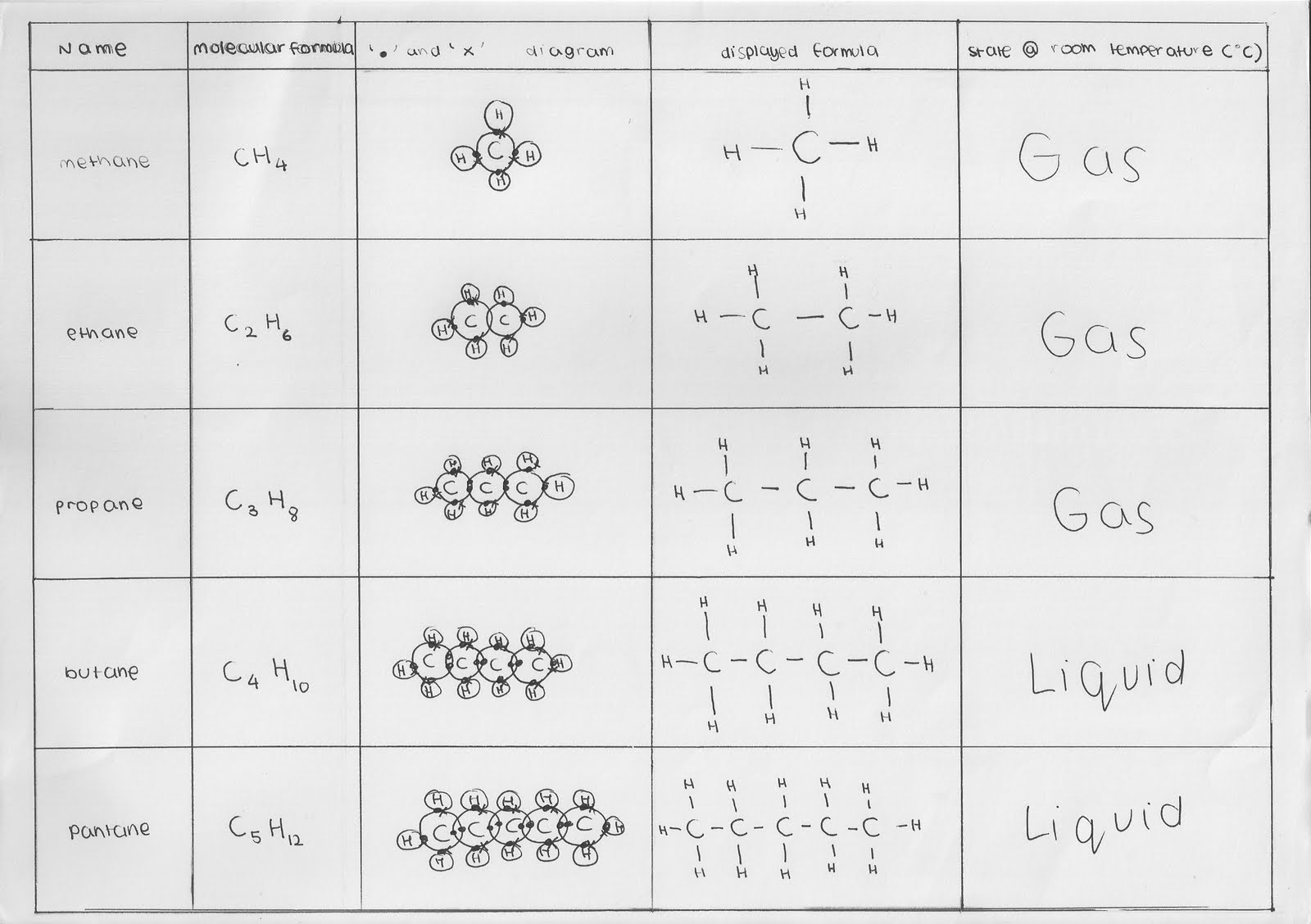
In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Alkenes have double bonds between carbons. This gives the parent name for the alkane 2.
Alkynes Organic Chemistry I Form Three Chemistry
The straight chain alkanes share the same general formula:
Let's look at the members of the alkane, alkene, and alkyne family and see what is their general molecular formula.
The name olefin is derived from the greek word olefin gas, which means oil forming. Methane gas is the first member of the homologous series of alkanes. This formula can be used. A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2:
For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each.
“alkanes & alkenes in chemistry” is an interactive app for students to learn about the alkanes, alkenes, reactions of alkenes, alkane to alkene, organic chemistry, alkene formula, alkynes in an easy and engrossing way by visualizing the colorful images. C x n h 2 n + 2. Alkene alkyne four major additions: In this regard, what are the first 10 alkynes?
Alkanes have the general formula of c n h 2n + 2 where n is the number of carbon atoms.
Alkanes have the general formula c n h 2n+2 and can be subdivided into the following three groups: The next few members are liquids and the higher ones are solids. Ethylene, propylene and α − b u t y l e n e are gases. Thus it is known as unsaturated hydrocarbons.
1) addition of hydrogen halides 2) halogenation :
We offer quizzes, questions, instructional videos. The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound. Reaction in which halogen is introduced into a molecule 3) hydration : Naming alkanes / alkenes / alkynes
The longest continuous chain should include both the carbon atoms of the triple bond.
Alkenes resemble alkanes in most of their physical properties. The general formula of the homologous series of alkanes is cnh2n + 2 where n is an integer. Alkenes are often used as a synonym of olefin. Except for ethylene, all other alkenes are colourless and odourless.
Ethylene is a colourless gas with a sickly sweet odour.
Alkenes have the general formula c n h 2n. Alkanes, alkenes and alkynes mcqs alkanes have single bonds between carbons in a hydrocarbon. And alkynes have triple bonds between carbons. The general formula is cnh2n, the same as cycloalkanes.
The general formula of alkenes are c n h 2n in comparison to alkanes with general formula c n h 2n+2.
\[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two. Discusses how to write the structural formula of the hydrocarbons alkanes, alkenes and alkynes. First member is ethyne c 2 h 2. What is the molecular formula of a 32 carbon alkyne?
The alkanes are also called as paraffins.
Acetylene is the simplest alkyne with the formula as c 2 h 2. 2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to: A quick way to recognize an alkane is the general formula: Alkanes can be obtained by hydrogenation of alkenes by means of hydrogen and catalyst.
For example, an alkane with 2(n) carbon atoms, will have 6 (2n+2) hydrogen atoms.
Use the number obtained by application of rule 2 to designate the position of the branched chain 4. A quick way to recognize an alkene is its general formula: You can use these generic formulas to predict the formula for any alkane, alkene, or alkyne. Number the parent chain beginning with the end of the chain nearer the branched chain 3.
C x n h 2 n + 2.
This gives them a general formula : Alkynes contain four hydrogen atoms less than corresponding alkanes and two hydrogen atoms less than corresponding alkenes and have the general formula c n h 2 n − 2. When two or more branched chains are present, give each branched. A) alkenes that contain 4 carbon atoms (three possible) b) cyclic alkenes that contain 4 carbon atoms (three possible) c) alkynes that contain 4 carbon atoms (two possible, neither of them are cyclic alkynes) 2.
Also, what are the general formulas for alkanes alkenes and alkynes?
Alkanes have the general formula c n h 2n+2. Reaction in which the elements of water (h and oh) are Alkenes are unsaturated hydrocarbons having a double bond between the carbon atoms. Alkenes are hydrocarbons that contain carbon carbon double bond (c=c).
Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes.